Stability of Cucumber Necrosis Virus at the Quasi-6-Fold Axis Affects Zoospore Transmission
- PMID: 28724762
- PMCID: PMC5599764
- DOI: 10.1128/JVI.01030-17
Stability of Cucumber Necrosis Virus at the Quasi-6-Fold Axis Affects Zoospore Transmission
Abstract
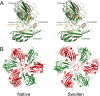
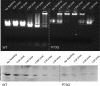
Cucumber necrosis virus (CNV) is a member of the genus Tombusvirus and has a monopartite positive-sense RNA genome. CNV is transmitted in nature via zoospores of the fungus Olpidium bornovanus As with other members of the Tombusvirus genus, the CNV capsid swells when exposed to alkaline pH and EDTA. We previously demonstrated that a P73G mutation blocks the virus from zoospore transmission while not significantly affecting replication in plants (K. Kakani, R. Reade, and D. Rochon, J Mol Biol 338:507-517, 2004, https://doi.org/10.1016/j.jmb.2004.03.008). P73 lies immediately adjacent to a putative zinc binding site (M. Li et al., J Virol 87:12166-12175, 2013, https://doi.org/10.1128/JVI.01965-13) that is formed by three icosahedrally related His residues in the N termini of the C subunit at the quasi-6-fold axes. To better understand how this buried residue might affect vector transmission, we determined the cryo-electron microscopy structure of wild-type CNV in the native and swollen state and of the transmission-defective mutant, P73G, under native conditions. With the wild-type CNV, the swollen structure demonstrated the expected expansion of the capsid. However, the zinc binding region at the quasi-6-fold at the β-annulus axes remained intact. By comparison, the zinc binding region of the P73G mutant, even under native conditions, was markedly disordered, suggesting that the β-annulus had been disrupted and that this could destabilize the capsid. This was confirmed with pH and urea denaturation experiments in conjunction with electron microscopy analysis. We suggest that the P73G mutation affects the zinc binding and/or the β-annulus, making it more fragile under neutral/basic pH conditions. This, in turn, may affect zoospore transmission.IMPORTANCECucumber necrosis virus (CNV), a member of the genus Tombusvirus, is transmitted in nature via zoospores of the fungus Olpidium bornovanus While a number of plant viruses are transmitted via insect vectors, little is known at the molecular level as to how the viruses are recognized and transmitted. As with many spherical plant viruses, the CNV capsid swells when exposed to alkaline pH and EDTA. We previously demonstrated that a P73G mutation that lies inside the capsid immediately adjacent to a putative zinc binding site (Li et al., J Virol 87:12166-12175, 2013, https://doi.org/10.1128/JVI.01965-13) blocks the virus from zoospore transmission while not significantly affecting replication in plants (K. Kakani, R. Reade, and D. Rochon, J Mol Biol 338:507-517, 2004, https://doi.org/10.1016/j.jmb.2004.03.008). Here, we show that the P73G mutant is less stable than the wild type, and this appears to be correlated with destabilization of the β-annulus at the icosahedral 3-fold axes. Therefore, the β-annulus appears not to be essential for particle assembly but is necessary for interactions with the transmission vector.
Keywords: RNA virus; cryo-EM; protein structure-function.
Copyright © 2017 American Society for Microbiology.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

