Histopathology of Veins Obtained at Hemodialysis Arteriovenous Fistula Creation Surgery
- PMID: 28724774
- PMCID: PMC5619951
- DOI: 10.1681/ASN.2016050598
Histopathology of Veins Obtained at Hemodialysis Arteriovenous Fistula Creation Surgery
Abstract
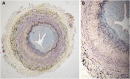
Stenosis from venous neointimal hyperplasia is common in native arteriovenous fistulas (AVFs). However, the preexisting histologic characteristics of veins at fistula creation, and associations thereof with baseline patient factors, have not been well characterized. In this study, we conducted histologic analysis of a segment of the vein used for anastomosis creation, obtained during AVF creation from 554 of the 602 participants in the multicenter Hemodialysis Fistula Maturation Cohort Study. We quantified intimal and medial areas and lengths of the internal and external elastic lamina by morphometry and assessed venous wall cells by immunohistochemistry, extracellular matrix with Movat stain, and calcium deposition by alizarin red stain. We also studied a representative subset of veins for markers of monocyte/macrophage content, cell proliferation, apoptosis, and neoangiogenesis. Neointima occupied >20% of the lumen in 57% of fully circumferential vein samples, and neointimal hyperplasia associated positively with age and inversely with black race. The neointima was usually irregularly thickened, sometimes concentric, and contained α-smooth muscle actin-expressing cells of smooth muscle or myofibroblast origin. Proteoglycans admixed with lesser amounts of collagen constituted the predominant matrix in the neointima. In 82% of vein samples, the media of vessel walls contained large aggregates of collagen. A minority of veins expressed markers of inflammation, cell proliferation, cell death, calcification, or neoangiogenesis. In conclusion, we observed preexisting abnormalities, including neointimal hyperplasia and prominent accumulation of extracellular matrix, in veins used for AVF creation from a substantial proportion of this cohort.
Keywords: arteriovenous access; arteriovenous fistula; dialysis access; pathology.
Copyright © 2017 by the American Society of Nephrology.
Figures








Comment in
-
Moving Beyond the Assumed: Improving Fistula Success Rates.J Am Soc Nephrol. 2017 Oct;28(10):2827-2829. doi: 10.1681/ASN.2017060663. Epub 2017 Jul 21. J Am Soc Nephrol. 2017. PMID: 28733368 Free PMC article. No abstract available.
References
-
- Dixon BS: Why don’t fistulas mature? Kidney Int 70: 1413–1422, 2006 - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

