Pediatric and adult dilated cardiomyopathy represent distinct pathological entities
- PMID: 28724792
- PMCID: PMC5518561
- DOI: 10.1172/jci.insight.94382
Pediatric and adult dilated cardiomyopathy represent distinct pathological entities
Abstract
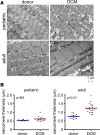
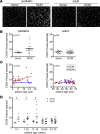
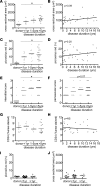
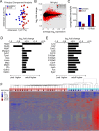
Pediatric dilated cardiomyopathy (DCM) is the most common indication for heart transplantation in children. Despite similar genetic etiologies, medications routinely used in adult heart failure patients do not improve outcomes in the pediatric population. The mechanistic basis for these observations is unknown. We hypothesized that pediatric and adult DCM comprise distinct pathological entities, in that children do not undergo adverse remodeling, the target of adult heart failure therapies. To test this hypothesis, we examined LV specimens obtained from pediatric and adult donor controls and DCM patients. Consistent with the established pathophysiology of adult heart failure, adults with DCM displayed marked cardiomyocyte hypertrophy and myocardial fibrosis compared with donor controls. In contrast, pediatric DCM specimens demonstrated minimal cardiomyocyte hypertrophy and myocardial fibrosis compared with both age-matched controls and adults with DCM. Strikingly, RNA sequencing uncovered divergent gene expression profiles in pediatric and adult patients, including enrichment of transcripts associated with adverse remodeling and innate immune activation in adult DCM specimens. Collectively, these findings reveal that pediatric and adult DCM represent distinct pathological entities, provide a mechanistic basis to explain why children fail to respond to adult heart failure therapies, and suggest the need to develop new approaches for pediatric DCM.
Keywords: Cardiology.
Conflict of interest statement
Figures


References
-
- Goldberg LR. In the clinic. Heart failure. Ann Intern Med. 2010;152(11):ITC61–15; quiz ITC616. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

