Increasing the level of peroxisome proliferator-activated receptor γ coactivator-1α in podocytes results in collapsing glomerulopathy
- PMID: 28724797
- PMCID: PMC5518556
- DOI: 10.1172/jci.insight.92930
Increasing the level of peroxisome proliferator-activated receptor γ coactivator-1α in podocytes results in collapsing glomerulopathy
Abstract
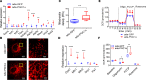
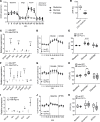
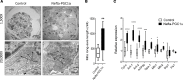
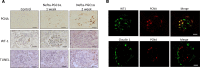
Inherited and acquired mitochondrial defects have been associated with podocyte dysfunction and chronic kidney disease (CKD). Peroxisome proliferator-activated receptor γ coactivator-1α (PGC1α) is one of the main transcriptional regulators of mitochondrial biogenesis and function. We hypothesized that increasing PGC1α expression in podocytes could protect from CKD. We found that PGC1α and mitochondrial transcript levels are lower in podocytes of patients and mouse models with diabetic kidney disease (DKD). To increase PGC1α expression, podocyte-specific inducible PGC1α-transgenic mice were generated by crossing nephrin-rtTA mice with tetO-Ppargc1a animals. Transgene induction resulted in albuminuria and glomerulosclerosis in a dose-dependent manner. Expression of PGC1α in podocytes increased mitochondrial biogenesis and maximal respiratory capacity. PGC1α also shifted podocytes towards fatty acid usage from their baseline glucose preference. RNA sequencing analysis indicated that PGC1α induced podocyte proliferation. Histological lesions of mice with podocyte-specific PGC1α expression resembled collapsing focal segmental glomerular sclerosis. In conclusion, decreased podocyte PGC1α expression and mitochondrial content is a consistent feature of DKD, but excessive PGC1α alters mitochondrial properties and induces podocyte proliferation and dedifferentiation, indicating that there is likely a narrow therapeutic window for PGC1α levels in podocytes.
Keywords: Metabolism; Nephrology.
Figures





References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

