Partially exhausted tumor-infiltrating lymphocytes predict response to combination immunotherapy
- PMID: 28724802
- PMCID: PMC5518562
- DOI: 10.1172/jci.insight.93433
Partially exhausted tumor-infiltrating lymphocytes predict response to combination immunotherapy
Abstract
Background: Programmed death 1 (PD-1) inhibition activates partially exhausted cytotoxic T lymphocytes (peCTLs) and induces tumor regression. We previously showed that the peCTL fraction predicts response to anti-PD-1 monotherapy. Here, we sought to correlate peCTL and regulatory T lymphocyte (Treg) levels with response to combination immunotherapy, and with demographic/disease characteristics, in metastatic melanoma patients.
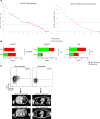
Methods: Pretreatment melanoma samples underwent multiparameter flow cytometric analysis. Patients were treated with anti-PD-1 monotherapy or combination therapy, and responses determined by Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST v1.1) criteria. peCTL and Treg levels across demographic/disease variables were compared. Low versus high peCTL (≤20% vs. >20%) were defined from a previous study.
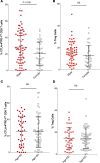
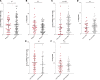
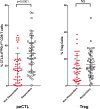
Results: One hundred and two melanoma patients were identified. The peCTL fraction was higher in responders than nonresponders. Low peCTL correlated with female sex and liver metastasis, but not with lactate dehydrogenase (LDH), tumor stage, or age. While overall response rates (ORRs) to anti-PD-1 monotherapy and combination therapy were similar in high-peCTL patients, low-peCTL patients given combination therapy demonstrated higher ORRs than those who received monotherapy. Treg levels were not associated with these factors nor with response.
Conclusion: In melanoma, pretreatment peCTL fraction is reduced in women and in patients with liver metastasis. In low-peCTL patients, anti-PD-1 combination therapy is associated with significantly higher ORR than anti-PD-1 monotherapy. Fewer tumor-infiltrating peCTLs may be required to achieve response to combination immunotherapy.
Trial registration: UCSF IRB Protocol 138510FUNDING. NIH DP2-AR068130, K08-AR062064, AR066821, and Burroughs Wellcome CAMS-1010934 (M.D.R.). Amoroso and Cook Fund, and the Parker Institute for Cancer Immunotherapy (A.I.D.).
Keywords: Cancer immunotherapy; Dermatology; Melanoma; Oncology; T cells.
Conflict of interest statement
Figures
References
-
- Rizvi NA, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16(3):257–265. doi: 10.1016/S1470-2045(15)70054-9. - DOI - PMC - PubMed

