X-ray crystallographic structure of a bacterial polysialyltransferase provides insight into the biosynthesis of capsular polysialic acid
- PMID: 28724897
- PMCID: PMC5517516
- DOI: 10.1038/s41598-017-05627-z
X-ray crystallographic structure of a bacterial polysialyltransferase provides insight into the biosynthesis of capsular polysialic acid
Abstract
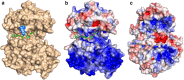
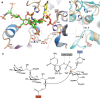
Polysialic acid (polySia) is a homopolymeric saccharide that is associated with some neuroinvasive pathogens and is found on selective cell types in their eukaryotic host. The presence of a polySia capsule on these bacterial pathogens helps with resistance to phagocytosis, cationic microbial peptides and bactericidal antibody production. The biosynthesis of bacterial polySia is catalysed by a single polysialyltransferase (PST) transferring sialic acid from a nucleotide-activated donor to a lipid-linked acceptor oligosaccharide. Here we present the X-ray structure of the bacterial PST from Mannheimia haemolytica serotype A2, thereby defining the architecture of this class of enzymes representing the GT38 family. The structure reveals a prominent electropositive groove between the two Rossmann-like domains forming the GT-B fold that is suitable for binding of polySia chain products. Complex structures of PST with a sugar donor analogue and an acceptor mimetic combined with kinetic studies of PST active site mutants provide insight into the principles of substrate binding and catalysis. Our results are the basis for a molecular understanding of polySia biosynthesis in bacteria and might assist the production of polysialylated therapeutic reagents and the development of novel antibiotics.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Regulation of capsular polysialic acid biosynthesis by temperature in Pasteurella haemolytica A2.FEBS Lett. 1999 Feb 26;445(2-3):325-8. doi: 10.1016/s0014-5793(99)00163-5. FEBS Lett. 1999. PMID: 10094482
-
Biochemical characterization of a polysialyltransferase from Mannheimia haemolytica A2 and comparison to other bacterial polysialyltransferases.PLoS One. 2013 Jul 26;8(7):e69888. doi: 10.1371/journal.pone.0069888. Print 2013. PLoS One. 2013. PMID: 23922842 Free PMC article.
-
Structure of human ST8SiaIII sialyltransferase provides insight into cell-surface polysialylation.Nat Struct Mol Biol. 2015 Aug;22(8):627-35. doi: 10.1038/nsmb.3060. Epub 2015 Jul 20. Nat Struct Mol Biol. 2015. PMID: 26192331
-
Polysialic acid: biosynthesis, novel functions and applications.Crit Rev Biochem Mol Biol. 2014 Nov-Dec;49(6):498-532. doi: 10.3109/10409238.2014.976606. Epub 2014 Nov 6. Crit Rev Biochem Mol Biol. 2014. PMID: 25373518 Review.
-
The Intrinsic Relationship Between Structure and Function of the Sialyltransferase ST8Sia Family Members.Curr Top Med Chem. 2017;17(21):2359-2369. doi: 10.2174/1568026617666170414150730. Curr Top Med Chem. 2017. PMID: 28413949 Review.
Cited by
-
Affinity-based covalent sialyltransferase probes enabled by ligand-directed chemistry.Chem Sci. 2025 Jan 13;16(7):3336-3344. doi: 10.1039/d4sc07184k. eCollection 2025 Feb 12. Chem Sci. 2025. PMID: 39845874 Free PMC article.
-
Transition transferases prime bacterial capsule polymerization.Nat Chem Biol. 2025 Jan;21(1):120-130. doi: 10.1038/s41589-024-01664-8. Epub 2024 Jul 1. Nat Chem Biol. 2025. PMID: 38951648 Free PMC article.
-
A New Family of Capsule Polymerases Generates Teichoic Acid-Like Capsule Polymers in Gram-Negative Pathogens.mBio. 2018 May 29;9(3):e00641-18. doi: 10.1128/mBio.00641-18. mBio. 2018. PMID: 29844111 Free PMC article.
-
3-O-Sulfation induces sequence-specific compact topologies in heparan sulfate that encode a dynamic sulfation code.Comput Struct Biotechnol J. 2022 Jul 18;20:3884-3898. doi: 10.1016/j.csbj.2022.07.013. eCollection 2022. Comput Struct Biotechnol J. 2022. PMID: 35891779 Free PMC article.
-
Processivity in Bacterial Glycosyltransferases.ACS Chem Biol. 2020 Jan 17;15(1):3-16. doi: 10.1021/acschembio.9b00619. Epub 2019 Dec 11. ACS Chem Biol. 2020. PMID: 31750644 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

