Metagenomics: The Next Culture-Independent Game Changer
- PMID: 28725217
- PMCID: PMC5495826
- DOI: 10.3389/fmicb.2017.01069
Metagenomics: The Next Culture-Independent Game Changer
Abstract
A trend towards the abandonment of obtaining pure culture isolates in frontline laboratories is at a crossroads with the ability of public health agencies to perform their basic mandate of foodborne disease surveillance and response. The implementation of culture-independent diagnostic tests (CIDTs) including nucleic acid and antigen-based assays for acute gastroenteritis is leaving public health agencies without laboratory evidence to link clinical cases to each other and to food or environmental substances. This limits the efficacy of public health epidemiology and surveillance as well as outbreak detection and investigation. Foodborne outbreaks have the potential to remain undetected or have insufficient evidence to support source attribution and may inadvertently increase the incidence of foodborne diseases. Next-generation sequencing of pure culture isolates in clinical microbiology laboratories has the potential to revolutionize the fields of food safety and public health. Metagenomics and other 'omics' disciplines could provide the solution to a cultureless future in clinical microbiology, food safety and public health. Data mining of information obtained from metagenomics assays can be particularly useful for the identification of clinical causative agents or foodborne contamination, detection of AMR and/or virulence factors, in addition to providing high-resolution subtyping data. Thus, metagenomics assays may provide a universal test for clinical diagnostics, foodborne pathogen detection, subtyping and investigation. This information has the potential to reform the field of enteric disease diagnostics and surveillance and also infectious diseases as a whole. The aim of this review will be to present the current state of CIDTs in diagnostic and public health laboratories as they relate to foodborne illness and food safety. Moreover, we will also discuss the diagnostic and subtyping utility and concomitant bias limitations of metagenomics and comparable detection techniques in clinical microbiology, food and public health laboratories. Early advances in the discipline of metagenomics, however, have indicated noteworthy challenges. Through forthcoming improvements in sequencing technology and analytical pipelines among others, we anticipate that within the next decade, detection and characterization of pathogens via metagenomics-based workflows will be implemented in routine usage in diagnostic and public health laboratories.
Keywords: antimicrobial resistance; culture-independent diagnostic test; food safety; metagenomics; molecular epidemiology; next-generation sequencing; public health; targeted-amplicon.
Figures

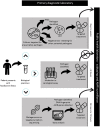
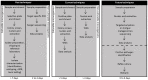
References
-
- Alfa M. J., Strang D., Tappia P. S., Graham M., Van Domselaar G., Forbes J. D., et al. (2017). A randomized trial to determine the impact of a digestion resistant starch composition on the gut microbiome in older and mid-age adults. Clin. Nutr. 10.1016/j.clnu.2017.03.025 [Epub ahead of print]. - DOI - PubMed
-
- Almonacid D. E., Kraal L., Ossandon F. J., Budovskaya Y. V., Cardenas J. P., Richman J., et al. (2016). 16S rRNA gene sequencing as a clinical diagnostic aid for gastrointestinal-related conditions. bioRxiv. 10.1101/084657 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

