Therapeutic strategies with oral fluoropyrimidine anticancer agent, S-1 against oral cancer
- PMID: 28725297
- PMCID: PMC5501734
- DOI: 10.1016/j.jdsr.2016.11.001
Therapeutic strategies with oral fluoropyrimidine anticancer agent, S-1 against oral cancer
Abstract
Oral cancer has been recognized as a tumor with low sensitivity to anticancer agents. However, introduction of S-1, an oral cancer agent is improving treatment outcome for patients with oral cancer. In addition, S-1, as a main drug for oral cancer treatment in Japan can be easily available for outpatients. In fact, S-1 exerts high therapeutic effects with acceptable side effects. Moreover, combined chemotherapy with S-1 shows higher efficacy than S-1 alone, and combined chemo-radiotherapy with S-1 exerts remarkable therapeutic effects. Furthermore, we should consider the combined therapy of S-1 and molecular targeting agents right now as these combinations were reportedly useful for oral cancer treatment. Here, we describe our findings related to S-1 that were obtained experimentally and clinically, and favorable therapeutic strategies with S-1 against oral cancer with bibliographic considerations.
Keywords: Chemo-radiotherapy; Chemotherapy; Oral cancer; S-1.
Figures

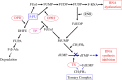

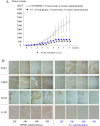




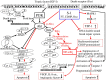
References
-
- Torre L.A., Bray F., Siegel R.L., Ferlay J., Lortet-Tieulent J., Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. - PubMed
-
- Japan Society for Head and Neck Cancer Registry Committee Report of head and neck cancer registry of Japan. Clinical statistics of registered patients, 2002. Jpn J Head Neck Cancer. 2006;32(Supplement):15–34.
-
- Cancer statistics in Japan—2014. 14th March, 2016. http://ganjoho.jp/reg_stat/statistics/brochure/backnumber/2014_jp.html.
-
- National Comprehensive Cancer Network (NCCN) 2012. NCCN clinical practice guidelines in oncology: head and neck cancers, Ver 1.http://www.nccn.org/clinical.asp 14th March, 2016. - PubMed
-
- Inagi K., Takahashi H., Okamoto M., Nakayama M., Makoshi T., Nagai H. Treatment effects in patients with squamous cell carcinoma of the oral cavity. Acta Otolaryngol Suppl. 2002;547:25–29. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

