Is contrast enhancement needed for diagnostic prostate MRI?
- PMID: 28725592
- PMCID: PMC5503975
- DOI: 10.21037/tau.2017.05.31
Is contrast enhancement needed for diagnostic prostate MRI?
Abstract
Prostate Imaging Reporting and Data System version 2 (PI-RADS v2) provides clinical guidelines for multiparametric magnetic resonance imaging (mpMRI) [T2-weighted imaging (T2WI), diffusion-weighted imaging (DWI) and dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI)] of prostate. However, DCE-MRI seems to show a limited contribution in prostate cancer (PCa) detection and management. In our experience, DCE-MRI, did not show significant change in diagnostic performance in addition to DWI and T2WI [biparametric MRI (bpMRI)] which represent the predominant sequences to detect suspected lesions in peripheral and transitional zone (TZ). In this article we reviewed the role of DCE-MRI also indicating the potential contribute of bpMRI approach (T2WI and DWI) and lesion volume evaluation in the diagnosis and management of suspected PCa.
Keywords: Prostate Imaging Reporting and Data System version 2 (PI-RADS v2); Prostate cancer (PCa); biparametric magnetic resonance imaging (bpMRI).
Conflict of interest statement
Conflicts of Interest: The authors have no conflicts of interest to declare.
Figures
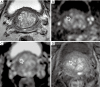
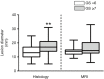
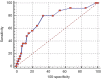
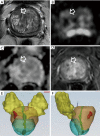

References
-
- Prostate Imaging Reporting and Data System (PI-RADS). Reston (VA): American College of Radiology. Available online: http://www.acr.org/Quality-Safety/ Resources/PIRADS/
-
- Abd-Alazeez M, Kirkham A, Ahmed HU, et al. Performance of multiparametric MRI in men at risk of prostate cancer before the first biopsy: a paired validating cohort study using template prostate mapping biopsies as the reference standard. Prostate Cancer Prostatic Dis 2014;17:40-6. 10.1038/pcan.2013.43 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
