Humanin G (HNG) protects age-related macular degeneration (AMD) transmitochondrial ARPE-19 cybrids from mitochondrial and cellular damage
- PMID: 28726777
- PMCID: PMC5550888
- DOI: 10.1038/cddis.2017.348
Humanin G (HNG) protects age-related macular degeneration (AMD) transmitochondrial ARPE-19 cybrids from mitochondrial and cellular damage
Abstract
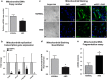
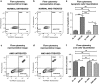
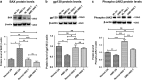
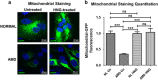
Age-related macular degeneration (AMD) ranks third among the leading causes of visual impairment with a blindness prevalence rate of 8.7%. Despite several treatment regimens, such as anti-angiogenic drugs, laser therapy, and vitamin supplementation, being available for wet AMD, to date there are no FDA-approved therapies for dry AMD. Substantial evidence implicates mitochondrial damage and retinal pigment epithelium (RPE) cell death in the pathogenesis of AMD. However, the effects of AMD mitochondria and Humanin G (HNG), a more potent variant of the mitochondrial-derived peptide (MDP) Humanin, on retinal cell survival have not been elucidated. In this study, we characterized mitochondrial and cellular damage in transmitochondrial cybrid cell lines that contain identical nuclei but possess mitochondria from either AMD or age-matched normal (Older-normal (NL)) subjects. AMD cybrids showed (1) reduced levels of cell viability, lower mtDNA copy numbers, and downregulation of mitochondrial replication/transcription genes and antioxidant enzyme genes; and (2) elevated levels of genes related to apoptosis, autophagy and ER-stress along with increased mtDNA fragmentation and higher susceptibility to amyloid-β-induced toxicity compared to NL cybrids. In AMD cybrids, HNG protected the AMD mitochondria, reduced pro-apoptosis gene and protein levels, upregulated gp130 (a component of the HN receptor complex), and increased the protection against amyloid-β-induced damage. In summary, in cybrids, damaged AMD mitochondria mediate cell death that can be reversed by HNG treatment. Our results also provide evidence of Humanin playing a pivotal role in protecting cells with AMD mitochondria. In the future, it may be possible that AMD patient's blood samples containing damaged mitochondria may be useful as biomarkers for this condition. In conclusion, HNG may be a potential therapeutic target for treatment of dry AMD, a debilitating eye disease that currently has no available treatment. Further studies are needed to establish HNG as a viable mitochondria-targeting therapy for dry AMD.
Conflict of interest statement
PC: Consultant and stockholder of CohBar Inc.; BDK: Clinical research: Alcon, Allergan, Apellis, Genentech, GSK, Ophthotech, Regeneron; Consultant: Alcon, Allergan, Catalyst, Genentech, Novartis, Ophthotech, Regeneron; Recipient: Allergan, Genentech, Novartis, Regeneron. The remaining authors declare no conflict of interest.
Figures




References
-
- Klein R, Chou CF, Klein BE, Zhang X, Meuer SM, Saaddine JB. Prevalence of age-related macular degeneration in the US population. Arch Ophthalmol 2011; 129: 75–80. - PubMed
-
- Au A, Parikh VS, Singh RP, Ehlers JP, Yuan A, Rachitskaya AV et al. Comparison of anti-VEGF therapies on fibrovascular pigment epithelial detachments in age-related macular degeneration. Br J Ophthalmol 2016; 101: 970–975. - PubMed
-
- McCusker MM, Durrani K, Payette MJ, Suchecki J. An eye on nutrition: the role of vitamins, essential fatty acids, and antioxidants in age-related macular degeneration, dry eye syndrome, and cataract. Clin Dermatol 2016; 34: 276–85. - PubMed
-
- Yu Dao-Yi, Cringle Stephen J, Yu Paula K, Su Er-Ning. Retinal energetics: its critical role in retinal physiology and pathology. Exp Rev Ophthalmol 2014; 6: 395–399.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

