Strain-induced skeletal rearrangement of a polycyclic aromatic hydrocarbon on a copper surface
- PMID: 28726802
- PMCID: PMC5524995
- DOI: 10.1038/ncomms16089
Strain-induced skeletal rearrangement of a polycyclic aromatic hydrocarbon on a copper surface
Abstract
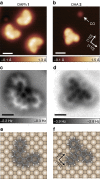
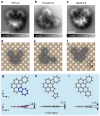
Controlling the structural deformation of organic molecules can drive unique reactions that cannot be induced only by thermal, optical or electrochemical procedures. However, in conventional organic synthesis, including mechanochemical procedures, it is difficult to control skeletal rearrangement in polycyclic aromatic hydrocarbons (PAHs). Here, we demonstrate a reaction scheme for the skeletal rearrangement of PAHs on a metal surface using high-resolution noncontact atomic force microscopy. By a combination of organic synthesis and on-surface cyclodehydrogenation, we produce a well-designed PAH-diazuleno[1,2,3-cd:1',2',3'-fg]pyrene-adsorbed flatly onto Cu(001), in which two azuleno moieties are highly strained by their mutual proximity. This local strain drives the rearrangement of one of the azuleno moieties into a fulvaleno moiety, which has never been reported so far. Our proposed thermally driven, strain-induced synthesis on surfaces will pave the way for the production of a new class of nanocarbon materials that conventional synthetic techniques cannot attain.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
-
- Wiberg K. B. The concept of strain in organic chemistry. Angew. Chem. Int. Ed. 25, 312–322 (1986).
-
- Piotti M. E. Ring opening metathesis polymerization. Curr. Opin. Solid State Mat. Sci. 4, 539–547 (1999).
-
- Beyer M. K. & Clausen-Schaumann H. Mechanochemistry: the mechanical activation of covalent bonds. Chem. Rev. 105, 2921–2948 (2005). - PubMed
-
- Ribas-Arino J. & Marx D. Covalent mechanochemistry: theoretical concepts and computational tools with applications to molecular nanomechanics. Chem. Rev. 112, 5412–5487 (2012). - PubMed
-
- Wang G. W. Mechanochemical organic synthesis. Chem. Soc. Rev. 42, 7668–7700 (2013). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

