Ribosomes are optimized for autocatalytic production
- PMID: 28726822
- PMCID: PMC7239375
- DOI: 10.1038/nature22998
Ribosomes are optimized for autocatalytic production
Abstract
Many fine-scale features of ribosomes have been explained in terms of function, revealing a molecular machine that is optimized for error-correction, speed and control. Here we demonstrate mathematically that many less well understood, larger-scale features of ribosomes-such as why a few ribosomal RNA molecules dominate the mass and why the ribosomal protein content is divided into 55-80 small, similarly sized segments-speed up their autocatalytic production.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

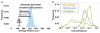
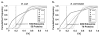





Comment in
-
Ribosomal Architecture: Constraints Imposed by the Need for Self-Production.Curr Biol. 2017 Aug 21;27(16):R798-R800. doi: 10.1016/j.cub.2017.06.080. Curr Biol. 2017. PMID: 28829964
References
-
- Rodnina MV, Wintermeyer W, Green R. Ribosomes Structure, Function, and Dynamics. Springer Science and Business Media; 2011.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

