A fluorescence-electrochemical study of carbon nanodots (CNDs) in bio- and photoelectronic applications and energy gap investigation
- PMID: 28726895
- PMCID: PMC5714648
- DOI: 10.1039/c7cp02875j
A fluorescence-electrochemical study of carbon nanodots (CNDs) in bio- and photoelectronic applications and energy gap investigation
Abstract
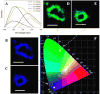
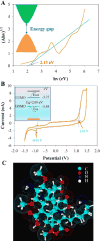
Carbon nanodots (CNDs) have attracted great attention due to their superior solubility, biocompatibility, tunable photoluminescence, and opto-electronic properties. This work describes a new fluorescence-based spectroelectrochemistry approach to simultaneously study the photoluminescence and wavelength dependent photocurrent of microwave synthesized CNDs. The fluorescence of CNDs shows selective quenching upon a reversible redox couple, ferricyanide/ferrocyanide, reaction during cyclic voltammetry. The CND modified gold slide electrode demonstrates wavelength dependent photocurrent generation during the fluorescence-electrochemical study, suggesting the potential application of CNDs in photoelectronics. UV-Vis absorption and electrochemistry are used to quantify the energy gap of the CNDs, and then to calibrate a Hückel model for CNDs' electronic energy levels. The Hückel (or tight binding) model treatment of an individual CND as a molecule combines the conjugated π states (C[double bond, length as m-dash]C) with the functional groups (C[double bond, length as m-dash]O, C-O, and COOH) associated with the surface electronic states. This experimental and theoretical investigation of CNDs provides a new perspective on the optoelectronic properties of CNDs and should aid in their development for practical use in biomedicine, chemical sensing, and photoelectric devices.
Figures



References
-
- Zhu S, Meng Q, Wang L, Zhang J, Song Y, Jin H, Zhang K, Sun H, Wang H, Yang B. Angewandte Chemie International Edition. 2013;52:3953–3957. - PubMed
-
- Lim SY, Shen W, Gao Z. Chemical Society Reviews. 2015;44:362–381. - PubMed
-
- Zheng XT, Ananthanarayanan A, Luo KQ, Chen P. Small. 2015;11:1620–1636. - PubMed
-
- Wang Y, Hu A. Journal of Materials Chemistry C. 2014;2:6921–6939.
-
- Li X, Rui M, Song J, Shen Z, Zeng H. Advanced Functional Materials. 2015;25:4929–4947.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

