Impact of Point-of-Care Xpert MTB/RIF on Tuberculosis Treatment Initiation. A Cluster-randomized Trial
- PMID: 28727491
- PMCID: PMC5649979
- DOI: 10.1164/rccm.201702-0278OC
Impact of Point-of-Care Xpert MTB/RIF on Tuberculosis Treatment Initiation. A Cluster-randomized Trial
Abstract
Rationale: Point-of-care (POC) diagnostics have the potential to reduce pretreatment loss to follow-up and delays to initiation of appropriate tuberculosis (TB) treatment.
Objectives: To evaluate the effect of a POC diagnostic strategy on initiation of appropriate TB treatment.
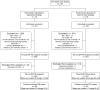
Methods: We conducted a cluster-randomized trial of adults with cough who were HIV positive and/or at high risk of drug-resistant TB. Two-week time blocks were randomized to two strategies: (1) Xpert MTB/RIF test (Cepheid, Sunnyvale, CA) performed at a district hospital laboratory or (2) POC Xpert MTB/RIF test performed at a primary health care clinic. All participants provided two sputum specimens: one for the Xpert test and the other for culture as a reference standard. The primary outcome was the proportion of participants with culture-positive pulmonary tuberculosis (PTB) initiated on appropriate TB treatment within 30 days.
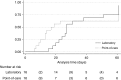
Measurements and main results: Between August 22, 2011, and March 1, 2013, 36 two-week blocks were randomized, and 1,297 individuals were enrolled (646 in the laboratory arm, 651 in the POC arm), 159 (12.4%) of whom had culture-positive PTB. The proportions of participants with culture-positive PTB initiated on appropriate TB treatment within 30 days were 76.5% in the laboratory arm and 79.5% in the POC arm (odds ratio, 1.13; 95% confidence interval, 0.51-2.53; P = 0.76; risk difference, 3.1%; 95% confidence interval, -16.2 to 10.1). The median time to initiation of appropriate treatment was 7 days (laboratory) versus 1 day (POC).
Conclusions: POC positioning of the Xpert test led to more rapid initiation of appropriate TB treatment. Achieving one-stop diagnosis and treatment for all people with TB will require simpler, more sensitive diagnostics and broader strengthening of health systems. Clinical trial registered with www.isrctn.com (ISRCTN 18642314) and www.sanctr.gov.za (DOH-27-0711-3568).
Keywords: clinical trial; drug-resistant tuberculosis; molecular diagnostics; point-of-care systems; tuberculosis.
Figures

Comment in
-
The Multistep Tuberculosis Diagnostic Cascade. More Than Meets the Eye.Am J Respir Crit Care Med. 2017 Oct 1;196(7):809-811. doi: 10.1164/rccm.201708-1600ED. Am J Respir Crit Care Med. 2017. PMID: 28846436 No abstract available.
References
-
- World Health Organization. Global tuberculosis report 2016. Geneva, Switzerland: World Health Organization; 2016 Oct 13 [accessed 2017 Jan 9]. Available from: http://apps.who.int/iris/bitstream/10665/250441/1/9789241565394-eng.pdf?....
-
- United Nations Development Programme; UN Economic Commission for Africa; African Union; African Development Bank Group. MDG report 2013: assessing progress in Africa toward the millennium development goals. Geneva, Switzerland: United Nations; 2013.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

