Hippocampus-dependent memory and allele-specific gene expression in adult offspring of alcohol-consuming dams after neonatal treatment with thyroxin or metformin
- PMID: 28727687
- PMCID: PMC5775940
- DOI: 10.1038/mp.2017.129
Hippocampus-dependent memory and allele-specific gene expression in adult offspring of alcohol-consuming dams after neonatal treatment with thyroxin or metformin
Abstract
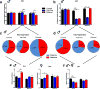
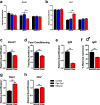
Fetal alcohol spectrum disorder (FASD), the result of fetal alcohol exposure (FAE), affects 2-11% of children worldwide, with no effective treatments. Hippocampus-based learning and memory deficits are key symptoms of FASD. Our previous studies show hypothyroxinemia and hyperglycemia of the alcohol-consuming pregnant rat, which likely affects fetal neurodevelopment. We administered vehicle, thyroxine (T4) or metformin to neonatal rats post FAE and rats were tested in the hippocampus-dependent contextual fear-conditioning paradigm in adulthood. Both T4 and metformin alleviated contextual fear memory deficit induced by FAE, and reversed the hippocampal expression changes in the thyroid hormone-inactivating enzyme, deiodinase-III (Dio3) and insulin-like growth factor 2 (Igf2), genes that are known to modulate memory processes. Neonatal T4 restored maternal allelic expressions of the imprinted Dio3 and Igf2 in the adult male hippocampus, while metformin restored FAE-caused changes in Igf2 expression only. The decreased hippocampal expression of DNA methyltransferase 1 (Dnmt1) that maintains the imprinting of Dio3 and Igf2 during development was normalized by both treatments. Administering Dnmt1 inhibitor to control neonates resulted in FAE-like deficits in fear memory and hippocampal allele-specific expression of Igf2, which were reversed by metformin. We propose that neonatal administration of T4 and metformin post FAE affect memory via elevating Dnmt1 and consequently normalizing hippocampal Dio3 and Igf2 expressions in the adult offspring. The present results indicate that T4 and metformin, administered during the neonatal period that is equivalent to the third trimester of human pregnancy, are potential treatments for FASD and conceivably for other neurodevelopmental disorders with cognitive deficits.
Conflict of interest statement
All authors reported no financial interests or potential conflicts of interest.
Figures

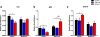

References
-
- Green PP, McKnight-Eily LR, Tan CH, Mejia R, Denny CH. Vital Signs: Alcohol-Exposed Pregnancies--United States, 2011–2013. MMWR Morb Mortal Wkly Rep. 2016;65:91–97. - PubMed
-
- Roozen S, Peters GJ, Kok G, Townend D, Nijhuis J, Curfs L. Worldwide Prevalence of Fetal Alcohol Spectrum Disorders: A Systematic Literature Review Including Meta-Analysis. Alcohol Clin Exp Res. 2016;40:18–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

