Intestinal Transit Time and Cortisol-Mediated Stress in Zebrafish
- PMID: 28727940
- PMCID: PMC5650717
- DOI: 10.1089/zeb.2017.1440
Intestinal Transit Time and Cortisol-Mediated Stress in Zebrafish
Abstract
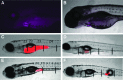
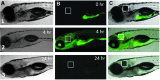
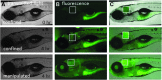
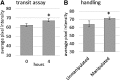
Intestinal motility, the spontaneous and rhythmic smooth muscle contraction, is a complex process that is regulated by overlapping and redundant regulatory mechanisms. Primary regulators intrinsic to the gastrointestinal tract include interstitial cells of Cajal, enteric neurons, and smooth muscle cells. Extrinsic primary regulators include the autonomic nervous system, immune system, and the endocrine system. Due to this complexity, a reductionist approach may be inappropriate if the ultimate goal is to understand motility regulation in vivo. Motility can be directly visualized in intact zebrafish, with intact regulatory systems, because larvae are transparent. Intestinal motility can therefore be measured in a complete system. However, the intestinal tract may respond to external influences, such as handling, which may invoke a stress response and influence intestinal transit. We used SR4G transgenic zebrafish, which express green fluorescent protein following activation of glucocorticoid receptors, and showed that handling required for the intestinal motility assay induces stress. Separate experiments showed that exogenous application of hydrocortisone did not influence intestinal transit, suggesting that handling may not interfere with transit measurements in intact zebrafish larvae. These experiments contribute to further development of the zebrafish model for intestinal motility research.
Keywords: intestine; motility; stress.
Conflict of interest statement
No Competing financial interests exist.
Figures
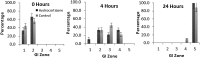

References
-
- Pitts SR, Niska RW, Xu J, Burt CW. National hospital ambulatory medical care survey: 2006 emergency department summary. Natl Health Stat Rep 2008;7:1–38 - PubMed
-
- Huizinga JD. Physiology and pathophysiology of the interstitial cell of Cajal: from bench to bedside. II. Gastric motility: lessons from mutant mice on slow waves and innervation. Am J Physiol Gastrointest Liver Physiol 2001;281:G1129–G1134 - PubMed
-
- Dodd A, Curtis PM, Williams LC, Love DR. Zebrafish: bridging the gap between development and disease. Hum Mol Genet 2000;9:2443–2449 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

