Patient-oriented education and medication management intervention for people with decompensated cirrhosis: study protocol for a randomized controlled trial
- PMID: 28728560
- PMCID: PMC5520368
- DOI: 10.1186/s13063-017-2075-4
Patient-oriented education and medication management intervention for people with decompensated cirrhosis: study protocol for a randomized controlled trial
Abstract
Background: People with decompensated cirrhosis require complex medical care and are often prescribed an intricate and frequently changing medication and lifestyle regimen. However, many patients mismanage their medications or have poor comprehension of their disease and self-management tasks. This can lead to harm, hospitalization, and death.
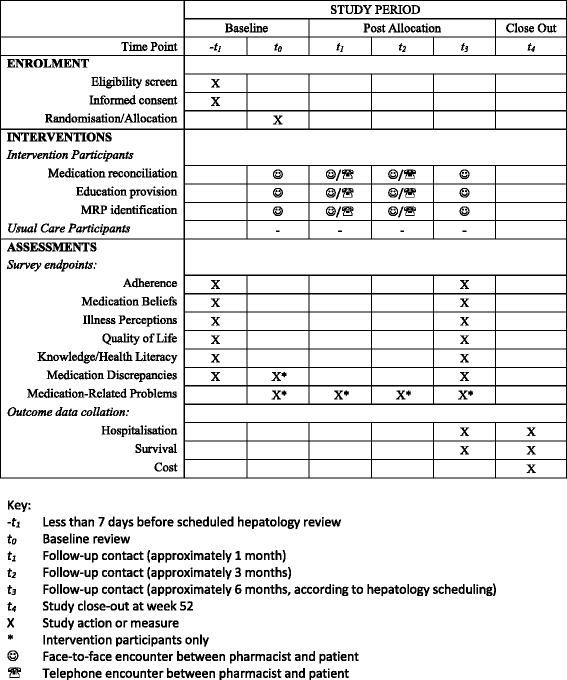
Methods/design: A patient-oriented education and medication management intervention has been developed for implementation at a tertiary hospital hepatology outpatient center in Queensland, Australia. Consenting patients with decompensated cirrhosis will be randomly allocated to education intervention or usual care treatment arms when they attend routine follow-up appointments. In the usual care arm, participants will be reviewed by their hepatologist according to the current model of care in the hepatology clinic. In the intervention arm, participants will be reviewed by a clinical pharmacist to receive the education and medication management intervention at baseline in addition to review by their hepatologist. Intervention participants will also receive three further educational contacts from the clinical pharmacist within the following 6-month period, in addition to routine hepatologist review that is scheduled within this time frame. All participants will be surveyed at baseline and follow-up (approximately 6 months post-enrollment). Validated questionnaire tools will be used to determine participant adherence, medication beliefs, illness perceptions, and quality of life. Patients' knowledge of dietary and lifestyle modifications, their current medications, and other clinical data will be obtained from the survey, patient interview, and medical records. Patient outcome data will be collected at 52 weeks.
Discussion: The intervention described within this protocol is ready to adapt and implement in hepatology ambulatory care centers globally. Investigation of potentially modifiable variables that may impact medication management, in addition to the effect of a clinical pharmacist-driven education and medication management intervention on modifying these variables, will provide valuable information for future management of these patients.
Trial registration: Australian and New Zealand Clinical Trial Registry identifier: ACTRN12616000780459 . Registered on 15 June 2016.
Keywords: Clinical pharmacist; Intervention study; Liver cirrhosis; Medication adherence; Medication errors; Medication reconciliation; Patient adherence; Patient education; Polypharmacy; Quality of life.
Conflict of interest statement
Ethics approval and consent to participate
Ethics approval was received for the study protocol from the Metro South Hospital and Health Service Human Research Ethics Committee (HREC/15/QPAH/688) and the University of Queensland Medical Research Ethics Committee (UQ2016000032) prior to commencement. Informed written consent will be obtained from each participant (and their formal carer/power of attorney as appropriate). A copy of the signed consent form will be filed in each patient’s medical record. The trial was retrospectively registered with the Australian and New Zealand Clinical Trial Registry on 15 June 2016 (ACTRN12616000780459).
Consent for publication
Not applicable, because individuals’ data are not identifiable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Hayward KH, Horsfall LU, Ruffin B, Cottrell WN, Chachay VS, Irvine KM, et al. Optimising care of patients with chronic disease: patient-oriented education may improve disease knowledge and self-management. Intern Med J. 47:8. doi:10.1111/imj.13505. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical