Robot Assisted Training for the Upper Limb after Stroke (RATULS): study protocol for a randomised controlled trial
- PMID: 28728602
- PMCID: PMC5520386
- DOI: 10.1186/s13063-017-2083-4
Robot Assisted Training for the Upper Limb after Stroke (RATULS): study protocol for a randomised controlled trial
Abstract
Background: Loss of arm function is a common and distressing consequence of stroke. We describe the protocol for a pragmatic, multicentre randomised controlled trial to determine whether robot-assisted training improves upper limb function following stroke.
Methods/design: Study design: a pragmatic, three-arm, multicentre randomised controlled trial, economic analysis and process evaluation.
Setting: NHS stroke services.
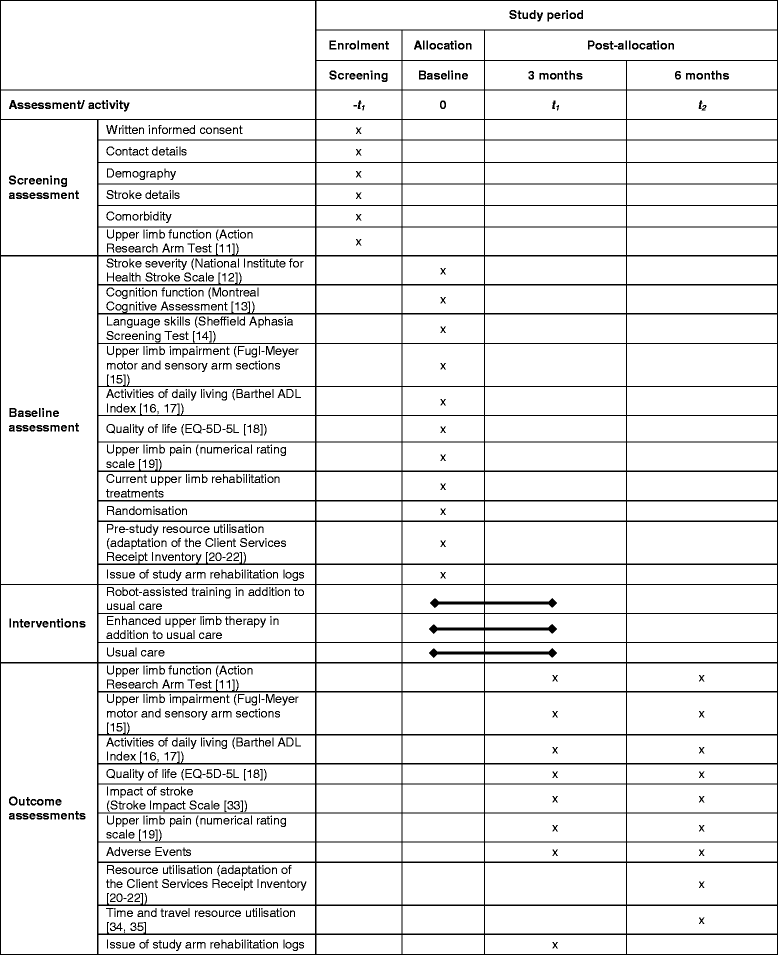
Participants: adults with acute or chronic first-ever stroke (1 week to 5 years post stroke) causing moderate to severe upper limb functional limitation. Randomisation groups: 1. Robot-assisted training using the InMotion robotic gym system for 45 min, three times/week for 12 weeks 2. Enhanced upper limb therapy for 45 min, three times/week for 12 weeks 3. Usual NHS care in accordance with local clinical practice Randomisation: individual participant randomisation stratified by centre, time since stroke, and severity of upper limb impairment.
Primary outcome: upper limb function measured by the Action Research Arm Test (ARAT) at 3 months post randomisation.
Secondary outcomes: upper limb impairment (Fugl-Meyer Test), activities of daily living (Barthel ADL Index), quality of life (Stroke Impact Scale, EQ-5D-5L), resource use, cost per quality-adjusted life year and adverse events, at 3 and 6 months. Blinding: outcomes are undertaken by blinded assessors. Economic analysis: micro-costing and economic evaluation of interventions compared to usual NHS care. A within-trial analysis, with an economic model will be used to extrapolate longer-term costs and outcomes. Process evaluation: semi-structured interviews with participants and professionals to seek their views and experiences of the rehabilitation that they have received or provided, and factors affecting the implementation of the trial.
Sample size: allowing for 10% attrition, 720 participants provide 80% power to detect a 15% difference in successful outcome between each of the treatment pairs. Successful outcome definition: baseline ARAT 0-7 must improve by 3 or more points; baseline ARAT 8-13 improve by 4 or more points; baseline ARAT 14-19 improve by 5 or more points; baseline ARAT 20-39 improve by 6 or more points.
Discussion: The results from this trial will determine whether robot-assisted training improves upper limb function post stroke.
Trial registration: ISRCTN, identifier: ISRCTN69371850 . Registered 4 October 2013.
Keywords: Arm; Cost-effectiveness analysis; Parallel process evaluation; RCT; Rehabilitation; Robotics; Stroke.
Conflict of interest statement
Ethics approval and consent to participate
The study sponsor is Newcastle upon Tyne Hospitals NHS Foundation Trust. The study is being conducted in accordance with Research Governance Framework for Health and Social Care [45]. Ethical approval was granted by the National Research Ethics Committee Sunderland (reference: 13/NE/0274). NHS Trust approvals have also been granted from Northumbria Healthcare NHS Foundation Trust, NHS Greater Glasgow and Clyde, Barking, Havering and Redbridge University Hospitals NHS Trust and London North West Hospitals NHS Trust. The following have NHS Trust approvals to act as spoke sites (Participant Identification Centres): Newcastle upon Tyne Hospitals NHS Foundation Trust, South Tyneside NHS Foundation Trust, Gateshead Health NHS Foundation Trust, County Durham and Darlington NHS Foundation Trust, North Middlesex University Hospital NHS Trust, University College London Hospitals NHS Foundation Trust, Barts Health NHS Foundation Trust, Mid Essex Hospital Services NHS Trust, North East London Foundation Trust and Royal Free London NHS Foundation Trust.
Written informed consent is obtained for all RATULS participants.
Consent for publication
Not applicable.
Competing interests
Dr. HI Krebs is a co-inventor in the MIT-held patents for the robotic devices used in this work. He holds equity positions in Bionik Laboratories, the company that manufactures this type of technology under license to MIT. All other authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Kwakkel G, Kollen BJ, van der Grond J, Prevo AJ. Probability of regaining dexterity in the flaccid upper limb: impact of severity of paresis and time since onset in acute stroke. Stroke. 2003;34:2181–6. doi: 10.1161/01.STR.0000087172.16305.CD. - DOI - PubMed
-
- Hallett M. Plasticity in the human motor system. Neuroscientist. 1999;5:324–32. doi: 10.1177/107385849900500518. - DOI
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical