Protection against cholera from killed whole-cell oral cholera vaccines: a systematic review and meta-analysis
- PMID: 28729167
- PMCID: PMC5639147
- DOI: 10.1016/S1473-3099(17)30359-6
Protection against cholera from killed whole-cell oral cholera vaccines: a systematic review and meta-analysis
Abstract
Background: Killed whole-cell oral cholera vaccines (kOCVs) are becoming a standard cholera control and prevention tool. However, vaccine efficacy and direct effectiveness estimates have varied, with differences in study design, location, follow-up duration, and vaccine composition posing challenges for public health decision making. We did a systematic review and meta-analysis to generate average estimates of kOCV efficacy and direct effectiveness from the available literature.
Methods: For this systematic review and meta-analysis, we searched PubMed, Embase, Scopus, and the Cochrane Review Library on July 9, 2016, and ISI Web of Science on July 11, 2016, for randomised controlled trials and observational studies that reported estimates of direct protection against medically attended confirmed cholera conferred by kOCVs. We included studies published on any date in English, Spanish, French, or Chinese. We extracted from the published reports the primary efficacy and effectiveness estimates from each study and also estimates according to number of vaccine doses, duration, and age group. The main study outcome was average efficacy and direct effectiveness of two kOCV doses, which we estimated with random-effect models. This study is registered with PROSPERO, number CRD42016048232.
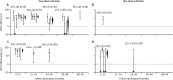
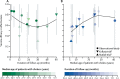
Findings: Seven trials (with 695 patients with cholera) and six observational studies (217 patients with cholera) met the inclusion criteria, with an average two-dose efficacy of 58% (95% CI 42-69, I2=58%) and effectiveness of 76% (62-85, I2=0). Average two-dose efficacy in children younger than 5 years (30% [95% CI 15-42], I2=0%) was lower than in those 5 years or older (64% [58-70], I2=0%; p<0·0001). Two-dose efficacy estimates of kOCV were similar during the first 2 years after vaccination, with estimates of 56% (95% CI 42-66, I2=45%) in the first year and 59% (49-67, I2=0) in the second year. The efficacy reduced to 39% (13 to 57, I2=48%) in the third year, and 26% (-46 to 63, I2=74%) in the fourth year.
Interpretation: Two kOCV doses provide protection against cholera for at least 3 years. One kOCV dose provides at least short-term protection, which has important implications for outbreak management. kOCVs are effective tools for cholera control.
Funding: The Bill & Melinda Gates Foundation.
Copyright This is an Open Access article published under the CC BY 3.0 IGO license which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. In any use of this article, there should be no suggestion that WHO endorses any specific organisation, products, or services. The use of the WHO logo is not permitted. This notice should be preserved along with the article's original URL.
Figures

Comment in
-
Oral cholera vaccines: exploring the farrago of evidence.Lancet Infect Dis. 2017 Oct;17(10):1012-1013. doi: 10.1016/S1473-3099(17)30420-6. Epub 2017 Jul 17. Lancet Infect Dis. 2017. PMID: 28729166 No abstract available.
References
-
- WHO Weekly epidemiological record. 2 June 2017;22:301–320. http://apps.who.int/iris/bitstream/10665/255611/1/WER9222.pdf?ua=1 92. (accessed June 17, 2017).
-
- Saha A, Chowdhury MI, Khanam F. Safety and immunogenicity study of a killed bivalent (O1 and O139) whole-cell oral cholera vaccine Shanchol, in Bangladeshi adults and children as young as 1 year of age. Vaccine. 2011;29:8285–8292. - PubMed
-
- Desai SN, Akalu Z, Teferi M. Comparison of immune responses to a killed bivalent whole cell oral cholera vaccine between endemic and less endemic settings. Trop Med Int Health. 2016;21:194–201. - PubMed
-
- Jertborn M, Svennerholm AM, Holmgren J. Safety and immunogenicity of an oral recombinant cholera B subunit—whole cell vaccine in Swedish volunteers. Vaccine. 1992;10:130–132. - PubMed
-
- Taylor DN, Cárdenas V, Sanchez JL. Two-year study of the protective efficacy of the oral whole cell plus recombinant B subunit cholera vaccine in Peru. J Infect Dis. 2000;181:1667–1673. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

