A review of rate control in atrial fibrillation, and the rationale and protocol for the RATE-AF trial
- PMID: 28729311
- PMCID: PMC5588987
- DOI: 10.1136/bmjopen-2016-015099
A review of rate control in atrial fibrillation, and the rationale and protocol for the RATE-AF trial
Abstract
Background and objective: Atrial fibrillation (AF) is common and causes impaired quality of life, an increased risk of stroke and death as well as frequent hospital admissions. The majority of patients with AF require control of heart rate. In this article , we summarise the limited evidence from clinical trials that guides prescription, and present the rationale and protocol for a new randomised trial. As rate control has not yet been shown to reduce mortality, there is a clear need to compare the impact of therapy on quality of life, cardiac function and exercise capacity. Such a trial should concentrate on the long-term effects of treatment in the largest proportion of patients with AF, those with symptomatic permanent AF, with the aim of improving patient well-being.
Design and intervention: The RAte control Therapy Evaluation in permanent Atrial Fibrillation (RATE-AF) trial will enrol 160 participants with a prospective, randomised, open-label, blinded end point design comparing initial rate control with digoxin or bisoprolol. This will be the first head-to-head randomised trial of digoxin and beta-blockers in AF.
Participants: Recruited patients will be aged ≥60 years with permanent AF and symptoms of breathlessness (equivalent to New York Heart Association class II or above), with few exclusion criteria to maximise generalisability to routine clinical practice.
Outcome measures: The primary outcome is patient-reported quality of life, with secondary outcomes including echocardiographic ventricular function, exercise capacity and biomarkers of cellular and clinical response. Follow-up will occur at 6 and 12 months, with feasibility components to inform the design of a future trial powered to detect a difference in hospital admission. The RATE-AF trial will underpin an integrated approach to management including biomarkers, functions and symptoms that will guide future research into optimal, personalised rate control in patients with AF.
Ethics and dissemination: East Midlands-Derby Research Ethics Committee (16/EM/0178); peer-reviewed publications.
Trial registration: Clinicaltrials.gov: NCT02391337; ISRCTN: 95259705. Pre-results.
Keywords: Echocardiography; Protocols & guidelines; RATE-AF; atrial fibrillation; heart rate; quality of life.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2017. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: None of the authors report a conflict of interest. All authors have completed the ICMJE uniform disclosure form (www.icmje.org/coi_disclosure.pdf) and declare: DK reports grants from Menarini, during the conduct of the study; non-financial support from Daiichi Sankyo and personal fees from AtriCure, outside the submitted work. MC reports grants from the National Institute of Health Research, during the conduct of the study; and personal fees from Astella Pharma and Ferring Pharma, outside the submitted work. PK reports consulting fees and honoraria from Bayer Healthcare, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Medtronic, Pfizer and Servier, all outside the submitted work; research grants from Bristol-Myers Squibb, Pfizer, Cardiovascular Therapeutics, Daiichi Sankyo, Sanofi, St. Jude Medical, German Federal Ministry for Education and Research (BMBF), Fondation Leducq, German Research Foundation (DFG), European Union, British Heart Foundation and Medical Research Council UK, all outside the submitted work; and is listed on two patent applications on AF therapy and markers for AF, both outside the submitted work. GYHL has served as a consultant for Bayer, Astellas, Merck, AstraZeneca, Sanofi, BMS/Pfizer, Biotronik, Portola and Boehringer Ingelheim, and has been on the speaker’s bureau for Bayer, BMS/Pfizer, Boehringer Ingelheim and Sanofi Aventis. RPS is the President of the British Society of Echocardiography. JJD, MG, MS. JNT, SM, GS report no competing interests.
Figures
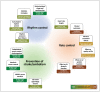

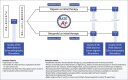
References
-
- Chiang CE, Naditch-Brûlé L, Murin J, et al. Distribution and risk profile of paroxysmal, persistent, and permanent atrial fibrillation in routine clinical practice: insight from the real-life global survey evaluating patients with atrial fibrillation international registry. Circ Arrhythm Electrophysiol 2012;5:632–9. 10.1161/CIRCEP.112.970749 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
