Comparative safety of antiepileptic drugs for neurological development in children exposed during pregnancy and breast feeding: a systematic review and network meta-analysis
- PMID: 28729328
- PMCID: PMC5642793
- DOI: 10.1136/bmjopen-2017-017248
Comparative safety of antiepileptic drugs for neurological development in children exposed during pregnancy and breast feeding: a systematic review and network meta-analysis
Abstract
Objectives: Compare the safety of antiepileptic drugs (AEDs) on neurodevelopment of infants/children exposed in utero or during breast feeding.
Design and setting: Systematic review and Bayesian random-effects network meta-analysis (NMA). MEDLINE, EMBASE and the Cochrane Central Register of Controlled Trials were searched until 27 April 2017. Screening, data abstraction and quality appraisal were completed in duplicate by independent reviewers.
Participants: 29 cohort studies including 5100 infants/children.
Interventions: Monotherapy and polytherapy AEDs including first-generation (carbamazepine, clobazam, clonazepam, ethosuximide, phenobarbital, phenytoin, primidone, valproate) and newer-generation (gabapentin, lamotrigine, levetiracetam, oxcarbazepine, topiramate, vigabatrin) AEDs. Epileptic women who did not receive AEDs during pregnancy or breast feeding served as the control group.
Primary and secondary outcome measures: Cognitive developmental delay and autism/dyspraxia were primary outcomes. Attention-deficit hyperactivity disorder, language delay, neonatal seizures, psychomotor developmental delay and social impairment were secondary outcomes.
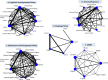
Results: The NMA on cognitive developmental delay (11 cohort studies, 933 children, 18 treatments) suggested that among all AEDs only valproate was statistically significantly associated with more children experiencing cognitive developmental delay compared with control (OR=7.40, 95% credible interval (CrI) 3.00 to 18.46). The NMA on autism (5 cohort studies, 2551 children, 12 treatments) suggested that oxcarbazepine (OR 13.51, CrI 1.28 to 221.40), valproate (OR 17.29, 95% CrI 2.40 to 217.60), lamotrigine (OR 8.88, CrI 1.28 to 112.00) and lamotrigine+valproate (OR 132.70, CrI 7.41 to 3851.00) were associated with significantly greater odds of developing autism compared with control. The NMA on psychomotor developmental delay (11 cohort studies, 1145 children, 18 treatments) found that valproate (OR 4.16, CrI 2.04 to 8.75) and carbamazepine+phenobarbital+valproate (OR 19.12, CrI 1.49 to 337.50) were associated with significantly greater odds of psychomotor delay compared with control.
Conclusions: Valproate alone or combined with another AED is associated with the greatest odds of adverse neurodevelopmental outcomes compared with control. Oxcarbazepine and lamotrigine were associated with increased occurrence of autism. Counselling is advised for women considering pregnancy to tailor the safest regimen.
Trial registration number: PROSPERO database (CRD42014008925).
Keywords: developmental delay; epilepsy; infants; knowledge synthesis; multiple treatment meta-analysis; pregnancy.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2017. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: AAV is funded by the Banting Postdoctoral Fellowship Program from the CIHR. SES is funded by a Tier 1 Canada Research Chair in Knowledge Translation. BH is funded by a CIHR/DSEN New Investigator Award in Knowledge Synthesis. BRH receives funding from the Alberta Heritage Foundation for Medical Research. ACT is funded by a Tier 2 Canada Research Chair in Knowledge Synthesis.
Figures

References
-
- Spina E, Perugi G. Antiepileptic drugs: indications other than epilepsy. Epileptic Disord 2004;6:57–75. - PubMed
-
- Harden CL, Pennell PB, Koppel BS, et al. Management issues for women with epilepsy--focus on pregnancy (an evidence-based review): III. Vitamin K, folic acid, blood levels, and breast-feeding: Report of the Quality Standards Subcommittee and Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Epilepsia 2009;50:1247–55. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
