Recommended Clinical Trial End Points for Dialysis Catheters
- PMID: 28729382
- PMCID: PMC5967684
- DOI: 10.2215/CJN.12011116
Recommended Clinical Trial End Points for Dialysis Catheters
Abstract
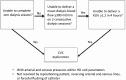
Central venous catheters are used frequently in patients on hemodialysis as a bridge to a permanent vascular access. They are prone to frequent complications, including catheter-related bloodstream infection, catheter dysfunction, and central vein obstruction. There is a compelling need to develop new drugs or devices to prevent central venous catheter complications. We convened a multidisciplinary panel of experts to propose standardized definitions of catheter end points to guide the design of future clinical trials seeking approval from the Food and Drug Administration. Our workgroup suggests diagnosing catheter-related bloodstream infection in catheter-dependent patients on hemodialysis with a clinical suspicion of infection (fever, rigors, altered mental status, or unexplained hypotension), blood cultures growing the same organism from the catheter hub and a peripheral vein (or the dialysis bloodline), and absence of evidence for an alternative source of infection. Catheter dysfunction is defined as the inability of a central venous catheter to (1) complete a single dialysis session without triggering recurrent pressure alarms or (2) reproducibly deliver a mean dialysis blood flow of >300 ml/min (with arterial and venous pressures being within the hemodialysis unit parameters) on two consecutive dialysis sessions or provide a Kt/V≥1.2 in 4 hours or less. Catheter dysfunction is defined only if it persists, despite attempts to reposition the patient, reverse the arterial and venous lines, or forcefully flush the catheter. Central vein obstruction is suspected in patients with >70% stenosis of a central vein by contrast venography or the equivalent, ipsilateral upper extremity edema, and an existing or prior history of a central venous catheter. There is some uncertainty about the specific criteria for these diagnoses, and the workgroup has also proposed future high-priority studies to resolve these questions.
Keywords: Bacteremia; Blood Culture; Catheter-Related Infections; Central Venous Catheters; Chills; Constriction; Edema; Humans; Pathologic; Phlebography; Uncertainty; United States; United States Food and Drug Administration; Veins; Venous Pressure; central vein; dialysis access; hypotension; renal dialysis; vascular access.
Copyright © 2018 by the American Society of Nephrology.
Figures
References
-
- Allon M: Current management of vascular access. Clin J Am Soc Nephrol 2: 786–800, 2007 - PubMed
-
- Collins AJ, Foley RN, Gilbertson DT, Chen SC: The state of chronic kidney disease, ESRD, and morbidity and mortality in the first year of dialysis. Clin J Am Soc Nephrol 4[Suppl 1]: S5–S11, 2009 - PubMed
-
- Allon M: Dialysis catheter-related bacteremia: Treatment and prophylaxis. Am J Kidney Dis 44: 779–791, 2004 - PubMed
-
- Lok CE, Mokrzycki MH: Prevention and management of catheter-related infection in hemodialysis patients. Kidney Int 79: 587–598, 2011 - PubMed
-
- Mokrzycki MH, Lok CE: Traditional and non-traditional strategies to optimize catheter function: Go with more flow. Kidney Int 78: 1218–1231, 2010 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases