A new approach for ratiometric in vivo calcium imaging of microglia
- PMID: 28729628
- PMCID: PMC5519759
- DOI: 10.1038/s41598-017-05952-3
A new approach for ratiometric in vivo calcium imaging of microglia
Abstract
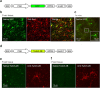
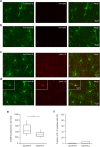
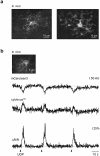
Microglia, resident immune cells of the brain, react to the presence of pathogens/danger signals with a large repertoire of functional responses including morphological changes, proliferation, chemotaxis, production/release of cytokines, and phagocytosis. In vitro studies suggest that many of these effector functions are Ca2+-dependent, but our knowledge about in vivo Ca2+ signalling in microglia is rudimentary. This is mostly due to technical reasons, as microglia largely resisted all attempts of in vivo labelling with Ca2+ indicators. Here, we introduce a novel approach, utilizing a microglia-specific microRNA-9-regulated viral vector, enabling the expression of a genetically-encoded ratiometric Ca2+ sensor Twitch-2B in microglia. The Twitch-2B-assisted in vivo imaging enables recording of spontaneous and evoked microglial Ca2+ signals and allows for the first time to monitor the steady state intracellular Ca2+ levels in microglia. Intact in vivo microglia show very homogenous and low steady state intracellular Ca2+ levels. However, the levels increase significantly after acute slice preparation and cell culturing along with an increase in the expression of activation markers CD68 and IL-1β. These data identify the steady state intracellular Ca2+ level as a versatile microglial activation marker, which is highly sensitive to the cell's environment.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

