Identification of a hybrid myocardial zone in the mammalian heart after birth
- PMID: 28729659
- PMCID: PMC5519540
- DOI: 10.1038/s41467-017-00118-1
Identification of a hybrid myocardial zone in the mammalian heart after birth
Abstract
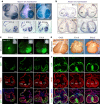
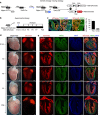
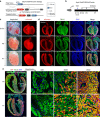
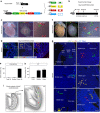
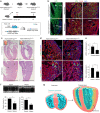
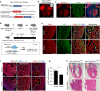
Noncompaction cardiomyopathy is characterized by the presence of extensive trabeculations, which could lead to heart failure and malignant arrhythmias. How trabeculations resolve to form compact myocardium is poorly understood. Elucidation of this process is critical to understanding the pathophysiology of noncompaction disease. Here we use genetic lineage tracing to mark the Nppa+ or Hey2+ cardiomyocytes as trabecular and compact components of the ventricular wall. We find that Nppa+ and Hey2+ cardiomyocytes, respectively, from the endocardial and epicardial zones of the ventricular wall postnatally. Interposed between these two postnatal layers is a hybrid zone, which is composed of cells derived from both the Nppa+ and Hey2+ populations. Inhibition of the fetal Hey2+ cell contribution to the hybrid zone results in persistence of excessive trabeculations in postnatal heart. Our findings indicate that the expansion of Hey2+ fetal compact component, and its contribution to the hybrid myocardial zone, are essential for normal formation of the ventricular walls.Fetal trabecular muscles in the heart undergo a poorly described morphogenetic process that results into a solidified compact myocardium after birth. Tian et al. show that cardiomyocytes in the fetal compact layer also contribute to this process, forming a hybrid myocardial zone that is composed of cells derived from both trabecular and compact layers.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




Comment in
-
Developmental biology: Formation of hybrid myocardial zone.Nat Rev Cardiol. 2017 Aug 16;14(9):504. doi: 10.1038/nrcardio.2017.125. Nat Rev Cardiol. 2017. PMID: 28812712 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

