Proteomic profiling of the weed feverfew, a neglected pollen allergen source
- PMID: 28729676
- PMCID: PMC5519751
- DOI: 10.1038/s41598-017-06213-z
Proteomic profiling of the weed feverfew, a neglected pollen allergen source
Abstract
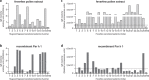
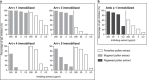
Feverfew (Parthenium hysterophorus), an invasive weed from the Asteraceae family, has been reported as allergen source. Despite its relevance, knowledge of allergens is restricted to a partial sequence of a hydroxyproline-rich glycoprotein. We aimed to obtain the entire sequence for recombinant production and characterize feverfew pollen using proteomics and immunological assays. Par h 1, a defensin-proline fusion allergen was obtained by cDNA cloning and recombinantly produced in E. coli. Using two complementary proteomic strategies, a total of 258 proteins were identified in feverfew pollen among those 47 proteins belonging to allergenic families. Feverfew sensitized patients' sera from India revealed IgE reactivity with a pectate lyase, PR-1 protein and thioredoxin in immonoblot. In ELISA, recombinant Par h 1 was recognized by 60 and 40% of Austrian and Indian sera, respectively. Inhibition assays demonstrated the presence of IgE cross-reactive Par h 1, pectate lyase, lipid-transfer protein, profilin and polcalcin in feverfew pollen. This study reveals significant data on the allergenic composition of feverfew pollen and makes recombinant Par h 1 available for cross-reactivity studies. Feverfew might become a global player in weed pollen allergy and inclusion of standardized extracts in routine allergy diagnosis is suggested in exposed populations.
Conflict of interest statement
B. Bohle has been supported by the Austrian Science Funds and by the Christian Doppler Laboratory for Immunomodulation. FF is a member of Scientific Advisory Boards (HAL Allergy, NL; AllergenOnline, USA) and has been supported by the Austrian Science Funds (FWF). GG received lecture fees from Thermo Fisher Scientific. The other authors declare that they have no relevant conflicts interest.
Figures



References
-
- Bajwa AA, Chauhan BS, Farooq M, Shabbir A, Adkins SW. What do we really know about alien plant invasion? A review of the invasion mechanism of one of the world’s worst weeds. Planta. 2016 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

