SYMPOSIUM REPORT: An Evidence-Based Approach to IBS and CIC: Applying New Advances to Daily Practice: A Review of an Adjunct Clinical Symposium of the American College of Gastroenterology Meeting October 16, 2016 • Las Vegas, Nevada
- PMID: 28729815
- PMCID: PMC5495029
SYMPOSIUM REPORT: An Evidence-Based Approach to IBS and CIC: Applying New Advances to Daily Practice: A Review of an Adjunct Clinical Symposium of the American College of Gastroenterology Meeting October 16, 2016 • Las Vegas, Nevada
Abstract
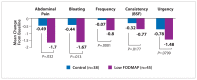
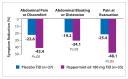
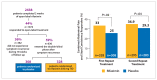
Many nonpharmacologic and pharmacologic therapies are available to manage irritable bowel syndrome (IBS) and chronic idiopathic constipation (CIC). The American College of Gastroenterology (ACG) regularly publishes reviews on IBS and CIC therapies. The most recent of these reviews was published by the ACG Task Force on the Management of Functional Bowel Disorders in 2014. The key objective of this review was to evaluate the efficacy of therapies for IBS or CIC compared with placebo or no treatment in randomized controlled trials. Evidence-based approaches to managing diarrhea-predominant IBS include dietary measures, such as a diet low in gluten and fermentable oligo-, di-, and monosaccharides and polyols (FODMAPs); loperamide; antispasmodics; peppermint oil; probiotics; tricyclic antidepressants; alosetron; eluxadoline, and rifaximin. Evidence-based approaches to managing constipation-predominant IBS and CIC include fiber, stimulant laxatives, polyethylene glycol, selective serotonin reuptake inhibitors, lubiprostone, and guanylate cyclase agonists. With the growing evidence base for IBS and CIC therapies, it has become increasingly important for clinicians to assess the quality of evidence and understand how to apply it to the care of individual patients.
Conflict of interest statement
Disclosure Dr Chey has received consultant/research support from Ardelyx, Ironwood Pharmaceuticals, Inc., and Vibrant. He is a consultant for Allergan, Inc., Ardelyx, IM HealthScience, Ironwood Pharmaceuticals, Inc., Prometheus, QOL Medical, Salix/Valeant Pharmaceuticals, and Vibrant. He has received honoraria from Ardelyx, IM HealthScience, Ironwood Pharmaceuticals, Inc., Prometheus, QOL Medical, and Salix/Valeant Pharmaceuticals. He has received other financial material support from CMO and My Total Health.
Figures

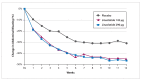

References
-
- Higgins PDR, Johanson JF. Epidemiology of constipation in North America: a systematic review. Am J Gastroenterol. 2004;99(4):750–759. - PubMed
-
- Saito YA, Schoenfeld P, Locke GR., III The epidemiology of irritable bowel syndrome in North America: a systematic review. Am J Gastroenterol. 2002;97(8):1910–1915. - PubMed
-
- Chang L, Lembo A, Sultan S. American Gastroenterological Association Institute Technical Review on the pharmacological management of irritable bowel syndrome. Gastroenterology. 2014;147(5):1149–1172.e2.. - PubMed
-
- Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: a clinical review. JAMA. 2015;313(9):949–958. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
