AN69ST membranes adsorb nafamostat mesylate and affect the management of anticoagulant therapy: a retrospective study
- PMID: 28729905
- PMCID: PMC5516335
- DOI: 10.1186/s40560-017-0244-x
AN69ST membranes adsorb nafamostat mesylate and affect the management of anticoagulant therapy: a retrospective study
Abstract
Background: In Japan, nafamostat mesylate (NM) is frequently used as an anticoagulant during continuous renal replacement therapy (CRRT). The dialyzer membrane AN69ST has been reported to adsorb NM and affect the management of anticoagulant therapy. However, the adsorbed amount has not yet been quantitatively assessed. Therefore, in this study, we evaluated the pre- and post-hemofilter prolongation of the activated clotting time (ACT) in patients with AN69ST and PS membranes. We also measured the adsorption of NM in three types of CRRT membranes using an experimental model.
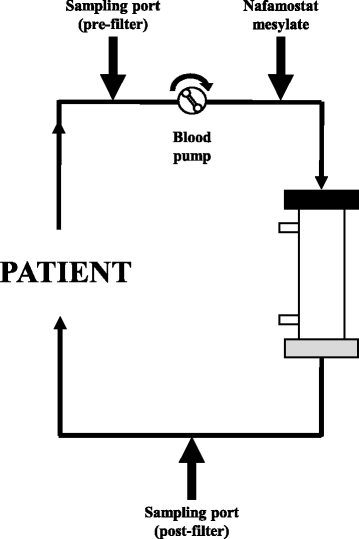
Methods: In a study of patients who underwent CRRT using AN69ST or PS membranes in 2015 at the Advanced Emergency and Critical Care Center, Okayama University Hospital, pre- and post-hemofilter ACT measurements were extracted retrospectively, and the difference was calculated. In addition, AN69ST (sepXiris100), PS (HEMOFEEL SHG-1.0), and PMMA membranes (HEMOFEEL CH-1.0N) were used in an in vitro model of a dialysis circuit, and the concentrations of NM were measured in pre- and post-hemofilter membranes and filtrates.
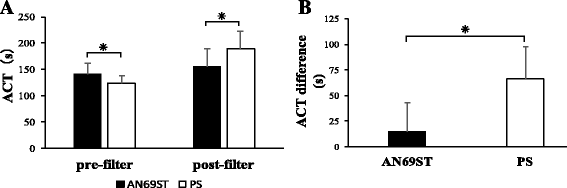
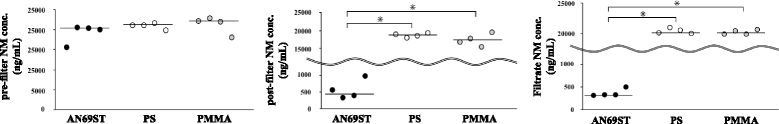
Results: The ACT difference was significantly lower in the group using AN69ST membranes (p < 0.01). In the in vitro model (n = 4) with adsorption and filtration, the post-hemofilter and filtrate concentrations of NM in AN69ST membranes were significantly lower than those in the PS and PMMA membranes (p < 0.01). The NM adsorption clearance of the AN69ST membrane was significantly higher than that of the PS and PMMA membranes.
Conclusions: The AN69ST membrane had higher NM adsorption than the PS and PMMA membranes. This may have resulted in the lower ACT difference in patients undergoing CRRT using the AN69ST membrane than in patients undergoing CRRT using PS or PMMA membranes.
Keywords: AN69ST; Adsorption; Anticoagulant therapy; Continuous renal replacement therapy; Nafamostat mesylate.
Conflict of interest statement
Ethical approval and consent to participate
This study was conducted in accordance with the principles of the Declaration of Helsinki because our research included a study that used human data. Our study was approved by the Ethics Committee of Okayama University Hospital (approval no. Ken1610-510). The study includes retrospective data with the highest privacy policy standards; therefore, the requirement of informed consent was waived.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
-
- Morabito S, Guzzo I, Solazzo A, Muzi L, Luciani R, Pierucci A. Continuous renal replacement therapies: anticoagulation in the critically ill at high risk of bleeding. J Nephrol. 2003;16:566–71. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

