Calcineurin inhibitor withdrawal or tapering for kidney transplant recipients
- PMID: 28730648
- PMCID: PMC6483545
- DOI: 10.1002/14651858.CD006750.pub2
Calcineurin inhibitor withdrawal or tapering for kidney transplant recipients
Abstract
Background: Calcineurin inhibitors (CNI) can reduce acute transplant rejection and immediate graft loss but are associated with significant adverse effects such as hypertension and nephrotoxicity which may contribute to chronic rejection. CNI toxicity has led to numerous studies investigating CNI withdrawal and tapering strategies. Despite this, uncertainty remains about minimisation or withdrawal of CNI.
Objectives: This review aimed to look at the benefits and harms of CNI tapering or withdrawal in terms of graft function and loss, incidence of acute rejection episodes, treatment-related side effects (hypertension, hyperlipidaemia) and death.
Search methods: We searched the Cochrane Kidney and Transplant Specialised Register to 11 October 2016 through contact with the Information Specialist using search terms relevant to this review. Studies contained in the Specialised Register are identified through search strategies specifically designed for CENTRAL, MEDLINE, and EMBASE; handsearching conference proceedings; and searching the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Selection criteria: All randomised controlled trials (RCTs) where drug regimens containing CNI were compared to alternative drug regimens (CNI withdrawal, tapering or low dose) in the post-transplant period were included, without age or dosage restriction.
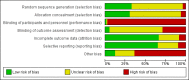
Data collection and analysis: Two authors independently assessed studies for eligibility, risk of bias, and extracted data. Results were expressed as risk ratio (RR) or mean difference (MD) with 95% confidence intervals (CI).
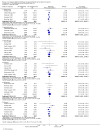
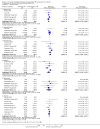
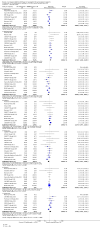
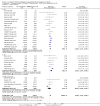
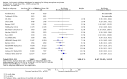
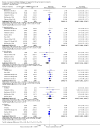
Main results: We included 83 studies that involved 16,156 participants. Most were open-label studies; less than 30% of studies reported randomisation method and allocation concealment. Studies were analysed as intent-to-treat in 60% and all pre-specified outcomes were reported in 54 studies. The attrition and reporting bias were unclear in the remainder of the studies as factors used to judge bias were reported inconsistently. We also noted that 50% (47 studies) of studies were funded by the pharmaceutical industry.We classified studies into four groups: CNI withdrawal or avoidance with or without substitution with mammalian target of rapamycin inhibitors (mTOR-I); and low dose CNI with or without mTOR-I. The withdrawal groups were further stratified as avoidance and withdrawal subgroups for major outcomes.CNI withdrawal may lead to rejection (RR 2.54, 95% CI 1.56 to 4.12; moderate certainty evidence), may make little or no difference to death (RR 1.09, 95% CI 0.96 to 1.24; moderate certainty), and probably slightly reduces graft loss (RR 0.85, 95% CI 0.74 to 0.98; low quality evidence). Hypertension was probably reduced in the CNI withdrawal group (RR 0.82, 95% CI 0.71 to 0.95; low certainty), while CNI withdrawal may make little or no difference to malignancy (RR 1.10, 95% CI 0.93 to 1.30; low certainty), and probably makes little or no difference to cytomegalovirus (CMV) (RR 0.87, 95% CI 0.52 to 1.45; low certainty)CNI avoidance may result in increased acute rejection (RR 2.16, 95% CI 0.85 to 5.49; low certainty) but little or no difference in graft loss (RR 0.96, 95% CI 0.79 to 1.16; low certainty). Late CNI withdrawal increased acute rejection (RR 3.21, 95% CI 1.59 to 6.48; moderate certainty) but probably reduced graft loss (RR 0.84, 95% CI 0.72 to 0.97, low certainty).Results were similar when CNI avoidance or withdrawal was combined with the introduction of mTOR-I; acute rejection was probably increased (RR 1.43; 95% CI 1.15 to 1.78; moderate certainty) and there was probably little or no difference in death (RR 0.96; 95% CI 0.69 to 1.36, moderate certainty). mTOR-I substitution may make little or no difference to graft loss (RR 0.94, 95% CI 0.75 to 1.19; low certainty), probably makes little of no difference to hypertension (RR 0.86, 95% CI 0.64 to 1.15; moderate), and probably reduced the risk of cytomegalovirus (CMV) (RR 0.60, 95% CI 0.44 to 0.82; moderate certainty) and malignancy (RR 0.69, 95% CI 0.47 to 1.00; low certainty). Lymphoceles were increased with mTOR-I substitution (RR 1.45, 95% CI 0.95 to 2.21; low certainty).Low dose CNI combined with mTOR-I probably increased glomerular filtration rate (GFR) (MD 6.24 mL/min, 95% CI 3.28 to 9.119; moderate certainty), reduced graft loss (RR 0.75, 95% CI 0.55 to 1.02; moderate certainty), and made little or no difference to acute rejection (RR 1.13 ; 95% CI 0.91 to 1.40; moderate certainty). Hypertension was decreased (RR 0.98, 95% CI 0.80 to 1.20; low certainty) as was CMV (RR 0.41, 95% CI 0.16 to 1.06; low certainty). Low dose CNI plus mTOR-I makes probably makes little of no difference to malignancy (RR 1.22, 95% CI 0.42 to 3.53; low certainty) and may make little of no difference to death (RR 1.16, 95% CI 0.71 to 1.90; moderate certainty).
Authors' conclusions: CNI avoidance increased acute rejection and CNI withdrawal increases acute rejection but reduced graft loss at least over the short-term. Low dose CNI with induction regimens reduced acute rejection and graft loss with no major adverse events, also in the short-term. The use of mTOR-I reduced CMV infections but increased the risk of acute rejection. These conclusions must be tempered by the lack of long-term data in most of the studies, particularly with regards to chronic antibody-mediated rejection, and the suboptimal methodological quality of the included studies.
Conflict of interest statement
Krishna M Karpe: none known
Girish S Talaulikar: none known
Giles Walters: none known.
Figures
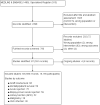






































Update of
References
References to studies included in this review
Abramowicz 2002 {published data only}
-
- Abramowicz D, Carmen Rial M, Vitko S, Castillo D, Manas D, Lao M, et al. Cyclosporine withdrawal from a mycophenolate mofetil‐containing immunosuppressive regimen: results of a five‐year, prospective, randomized study. Journal of the American Society of Nephrology 2005;16(7):2234‐40. [MEDLINE: ] - PubMed
-
- Abramowicz D, Manas D, Lao M, Vanrenterghem Y, Castillo D, Wijngaard P, et al. Cyclosporine withdrawal from a mycophenolate mofetil‐containing immunosuppressive regimen in stable kidney transplant recipients: a randomized, controlled study. Transplantation 2002;74(12):1725‐34. [MEDLINE: ] - PubMed
-
- Castillo D, Abramowicz D, Manas D, Lao M, Vanrenterghem Y, Barker D, et al. Benefits of cyclosporine (CsA) withdrawal in stable renal transplant recipients, receiving mycophenolate mofetil (MMF) and steroids (S): a multicenter, randomised, controlled study [abstract no: A3674]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):726A. [CENTRAL: CN‐00550730]
Alsina 1987 {published data only}
-
- Alsina J, Grino JM, Castelao AM, Sabate I, Mestre M, Gil‐Vernet S, et al. Low dose cyclosporine (CsA), ALG and steroids in first cadaveric renal transplants [abstract]. 10th International Congress of Nephrology; 1987 Jul 26‐31; London, UK. 1987:606. [CENTRAL: CN‐00583779]
-
- Grino JM, Castelao AM, Sabate I, Mestre M, Gil‐Vernet S, Andres E, et al. Low dose cyclosporine (CyA), ALG and steroids in first cadaveric renal transplants [abstract]. Kidney International 1988;34(2):301. [CENTRAL: CN‐00644138]
-
- Grino JM, Castelao AM, Sabate I, Mestre M, Gil‐Vernet S, Andres E, et al. Low‐dose cyclosporin (CsA), ALG and steroids in first cadaveric renal transplants [abstract]. Nephrology Dialysis Transplantation 1988;3(1):99‐100.
Andres 2009 {published data only}
-
- Andres A, Marcen R, Sanchez J, Valdes F, Errasti P, Sola R, et al. Comparative study of three different patterns of immunosuppression based on induction with basiliximab and low initial doses or delayed initiation of neoral in kidney transplant recipients at high risk of delayed graft function [abstract no: 1629]. American Journal of Transplantation 2005;5(Suppl 11):570. [CENTRAL: CN‐00644164]
-
- Andres A, Marcen R, Valdes F, Plumed JS, Sola R, Errasti P, et al. A randomized trial of basiliximab with three different patterns of cyclosporin A initiation in renal transplant from expanded criteria donors and at high risk of delayed graft function. Clinical Transplantation 2009;23(1):23‐32. [MEDLINE: ] - PubMed
-
- Marcen R, Andres A, Sanchez‐Plumed J, Valdes F, Errasti P, Sola R, et al. A randomized study to compare immunosuppression based on basiliximab induction and low initial doses or delayed neoral in kidney transplant recipients at high risk for delayed graft function [abstract no: PO‐266]. Transplant International 2005;18(Suppl 1):114.
APOLLO Study 2015 {published data only}
-
- Arns W, Rath T, Sommerer C, Reinke P, Haller H, Suwelack B, et al. Outcome on renal function of an everolimus based therapy after calcineurin inhibitor withdrawal in maintenance renal transplant recipients: 5 year data of the APOLLO trial [abstract]. Transplantation 2014;98(Suppl 1):145. [EMBASE: 71543988]
-
- Budde K, Rath T, Sommerer C, Haller H, Reinke P, Witzke O, et al. Renal, efficacy and safety outcomes following late conversion of kidney transplant patients from calcineurin inhibitor therapy to everolimus: the randomized APOLLO study. Clinical Nephrology 2015;83(1):11‐21. [MEDLINE: ] - PubMed
-
- Budde K, Sommerer C, Haller H, Suwelack B, May C, Paulus E, et al. Renal function of an everolimus based therapy after calcineurin inhibitor withdrawal in maintenance renal transplant recipients: 3 year data of the APOLLO trial [abstract no: 928]. American Journal of Transplantation 2012;12(Suppl S3):298. [EMBASE: 70746881]
-
- Budde K, Sommerer C, Rath T, Reinke P, Haller H, Witzke O, et al. Renal function to 5 years after late conversion of kidney transplant patients to everolimus: a randomized trial. Journal of Nephrology 2015;28(1):115‐23. [MEDLINE: ] - PubMed
-
- Budde K, Sommerer C, Reinke P, Haller H, Arns W, Witzke O, et al. Outcome on renal function of an everolimus based therapy after calcineurin inhibitor withdrawal in maintenance renal transplant recipients: 4 year data of the APOLLO Trial [abstract no: B396]. American Journal of Transplantation 2013;13(Suppl S5):311‐2. [EMBASE: 71057512]
Asberg 2006 {published data only}
-
- Asberg A, Midtvedt K, Line PD, Narverud J, Holdaas H, Jenssen T, et al. Calcineurin inhibitor avoidance with daclizumab, mycophenolate mofetil, and prednisolone in DR‐matched de novo kidney transplant recipients. Transplantation 2006;82(1):62‐8. [MEDLINE: ] - PubMed
-
- Asberg A, Midtvedt K, Line PD, Narverud J, Holdaas H, Jenssen T, et al. Calcineurin inhibitor‐avoidance with daclizumab, mycophenolate mofetil and prednisolone in DR matched de novo kidney transplant recipients [abstract no: SP737]. Nephrology Dialysis Transplantation 2006;21(Suppl 4):iv264. - PubMed
-
- Asberg A, Midtvedt K, Voytovich MH, Line PD, Narverud J, Reisaeter AV, et al. Calcineurin inhibitor effects on glucose metabolism and endothelial function following renal transplantation. Clinical Transplantation 2009;23(4):511‐8. [MEDLINE: ] - PubMed
-
- Asberg A, Midtvedt K, Voytovich MH, Line PD, Narverud J, Reisaeter AV, et al. Calcineurin inhibitor effects on glucose metabolism and endothelial function following renal transplantation [abstract no: 529]. American Journal of Transplantation 2008;8(Suppl 2):319. - PubMed
ASCERTAIN Study 2011 {published data only}
-
- Holdaas H, Midtvedt K, Seron D, O'Connell P, Thiagarajan CM, Fassett R, et al. Everolimus in maintenance renal transplant recipients: the ASCERTAIN Study [abstract no: P‐309]. Transplant International 2009;22(Suppl 2):172.
-
- Holdaas H, Midtvedt K, Seron D, O'Connell P, Thiagrajan C, Fassett R, et al. Assessment of everolimus in maintenance renal transplant recipients: the Ascertain Study [abstract no: 1646]. Transplantation 2008;86(Suppl 2):546. [CENTRAL: CN‐00740482]
-
- Holdaas H, Rostaing L, Midtvedt K, Seron D, Cole E, Chapman J, Fellstrom B, et al. Conversion of long‐term kidney transplant recipients from calcineurin inhibitor therapy to everolimus: a randomized, multicenter, 24‐month study.[Erratum appears in Transplantation. 2011 Nov 27;92(10):e61 Note: multiple investigator names added.]. Transplantation 2011;92(4):410‐8. [MEDLINE: ] - PubMed
-
- O'Connell P, Fassett R, Pilmore H, Chapman J, Hutchison B, Russ G, et al. Long‐term post transplantation switch to an everolimus‐based therapy with CNI elimination/minimization does not overall impact graft function: the ASCERTAIN study [abstract no: 8]. Transplantation Society of Australia & New Zealand (TSANZ). 29th Annual Scientific Meeting; 2011 Jun 29‐Jul 1; Canberra (ACT). 2011:42.
-
- O'Connell P, Fassett R, Pilmore H, Chapman J, Hutchison B, Russ G, et al. Post‐hoc analysis of the ASCERTAIN trial: everolimus based therapy with CNI elimination improves renal function in select populations [abstract no: 7]. Transplantation Society of Australia & New Zealand (TSANZ).29th Annual Scientific Meeting; 2011 Jun 29‐Jul 1; Canberra (ACT). 2011:41.
Baczkowska 2003 {published data only}
-
- Baczkowska T, Durlik M, Perkowska A, Sadowska A, Cieciura T, Nowacka‐Cieciura E, et al. Cytokines and growth factors serum level and renal allograft function (preliminary report) [Cytokiny i czynniki wzrostu w surowicy u biorcow nerki allogenicznej a losy przeszczepu (doniesienie wstepne)]. Polski Merkuriusz Lekarski 2003;15(88):356‐8. [MEDLINE: ] - PubMed
-
- Baczkowska T, Perkowska A, Cieciura T, Wierzbicki P, Klosowka D, Matlosz B, et al. Daclizumab allows for a protocol with low‐dose cyclosporine in low rejection‐risk kidney recipients ‐ preliminary data [abstract no: T435]. Nephrology Dialysis Transplantation 2002;17(Suppl 1):309. [CENTRAL: CN‐00400168]
-
- Baczkowska T, Perkowska‐Francka A, Durlik M, Cieciura T, Nowacka‐Cieciura E, Pazik J, et al. The role of the protocol biopsies in renal allograft recipients. Transplantation Proceedings 2003;35(6):2179‐81. [MEDLINE: ] - PubMed
-
- Baczkowska T, Perkowska‐Ptasinska A, Cieciura T, Pazik J, Nowacka‐Cieciura E, Lewandowski Z, et al. Untreated subclinical, borderline rejection in 12 and 36‐month protocol biopsies is not associated with progressive loss of GFR at 5‐year's follow‐up [abstract no: 1755]. Transplantation 2008;86(2 Suppl):581. [CENTRAL: CN‐00671803]
-
- Baczkowska T, Perkowska‐Ptasinska A, Lewandowski Z, Nowacka‐Cieciura E, Cieciura T, Pazik J, et al. Serum TGF‐b1 correlates with chronic histopathological lesions in protocol biopsies in kidney allograft recipients [abstract no: P354]. Transplantation 2004;78(2 Suppl):319. [CENTRAL: CN‐00583398] - PubMed
Bansal 2013 {published data only}
-
- Bansal D, Yadav AK, Kumar V, Minz M, Sakhuja V, Jha V. Deferred pre‐emptive switch from calcineurin inhibitor to sirolimus leads to improvement in GFR and expansion of t‐regulatory cell population: a randomized, controlled trial. PLoS ONE [Electronic Resource] 2013;8(10):e75591. [MEDLINE: ] - PMC - PubMed
Barsoum 2007 {published data only}
-
- Barsoum RS, Morsey AA, Iskander IR, Morgan MM, Fayad TM, Atalla NT, et al. The Cairo Kidney Center protocol for rapamycin‐based sequential immunosuppression in kidney transplant recipients: 2‐year outcomes. Experimental & Clinical Transplantation 2007;5(2):649‐57. [MEDLINE: ] - PubMed
Bechstein‐193 2013 {published data only}
-
- Bechstein W, Paczek L. A phase II, open‐label, concentration‐controlled, randomized 6‐month study of standard‐dose tacrolimus + sirolimus + corticosteroids compared to reduced‐dose tacrolimus + sirolimus + corticosteroids in renal allograft recipients [abstract]. American Journal of Transplantation 2002;2(Suppl 3):471. [CENTRAL: CN‐00415252]
-
- Bechstein WO, Paczek L, Wramner L, Squifflet JP, European Sirolimus‐Tacrolimus Study Group. An open‐label, concentration‐controlled, randomised 6‐month study of standard‐dose tacrolimus + sirolimus + steroids compared to reduced‐dose tacrolimus + sirolimus + steroids in renal allograft recipients [abstract]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):785. [CENTRAL: CN‐00444369]
-
- Bechstein WO, Paczek L, Wramner L, Squifflet JP, Zygmunt AJ, European Rapamune Tacrolimus Study Group. A comparative, randomized trial of concentration‐controlled sirolimus combined with reduced‐dose tacrolimus or standard‐dose tacrolimus in renal allograft recipients. Transplantation Proceedings 2013;45(6):2133‐40. [MEDLINE: ] - PubMed
-
- Paczek L, Bechstein W, Wramner L, Squifflet JP. A phase II, open‐label, concentration‐controlled, randomized 6‐month study of standard‐dose tacrolimus + sirolimus + corticosteroids compared to reduced‐dose tacrolimus + sirolimus + corticosteroids in renal allograft recipients [abstract]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002.
-
- Paczek L, Bechstein WO, Wramner L, Squifflet JP, European Sirolimus‐Tacrolimus Study Group. An open‐label, concentration‐controlled, randomised 6‐month study of standard‐dose tacrolimus + sirolimus +steroids compared to reduced‐dose tacrolimus + sirolimus + steroids in renal allograft recipients [abstract]. American Journal of Transplantation 2003;3(Suppl 5):464.
Bertoni 2007 {published data only}
-
- Bertoni E, Becherelli P, Salvadori M. Triple therapy with neoral, steroids and enteric‐coated mycophenolate acid vs everolimus: efficacy, side effects and pharmaco‐economic aspects. A monocentric experience [abstract no: F‐PO614]. Journal of the American Society of Nephrology 2007;18(Abstracts):234A. [CENTRAL: CN‐00716078]
-
- Rosati A, Bertoni E, Rosso G, Larti A, Mehmetaj A, Salvadori M. Triple therapy with Neoral, steroids and enteric coated mycophenolic acid vs everolimus: efficacy, side effects and pharmaco‐economic aspects. A monocentric experience [abstract no: SaP477]. Nephrology Dialysis Transplantation 2007;22(Suppl 6):vi396. [CENTRAL: CN‐00725011]
Bertoni 2011 {published data only}
-
- Bertoni E, Larti A, Farsetti S, Rosso G, Maria L, Zanazzi M. Cyclosporine (CyA) very low dose with everolimus (E) high dose is associated with better outcomes in renal transplant patients with respect to standard treatment with EC‐MPS (M) [abstract]. Transplant International 2009;22(Suppl 2):91.
-
- Bertoni E, Larti A, Rosso G, Zanazzi M, Maria L, Salvadori M. Good outcomes with cyclosporine very low exposure with everolimus high exposure in renal transplant patients. Journal of Nephrology 2011;24(5):613‐8. [MEDLINE: ] - PubMed
Budde 2007 {published data only}
-
- Budde K, Bosmans J, Zeier M, Sennesael J, Hopt U, Fischer WH, et al. Safety and efficacy of reduced or full dose of cyclosporine (neoral®) in combination with mycophenolate sodium (Myfortic®), basiliximab (Simulect®), and steroids in de novo kidney transplant recipients [abstract]. Transplantation 2004;78(2 Suppl):83. [CENTRAL: CN‐00527096]
-
- Budde K, Bosmans JL, Sennesael J, Zeier M, Hopt U, Fischer W, et al. Reduced cyclosporine exposure is safe and efficacious in combination with basiliximab, enteric‐coated mycophenolate‐sodium, and steroids [abstract no: 1195]. American Journal of Transplantation 2005;5(Suppl 11):461. [CENTRAL: CN‐00644165]
-
- Budde K, Bosmans JL, Sennesael J, Zeier M, Pisarski P, Schutz M, et al. Reduced‐exposure cyclosporine is safe and efficacious in de novo renal transplant recipients treated with enteric‐coated mycophenolic acid and basiliximab. Clinical Nephrology 2007;67(3):164‐75. [MEDLINE: ] - PubMed
-
- Budde K, Zeier M, Bosmans JL, Sennesael J, Glander P, Fischer W, et al. Reduced‐exposure cyclosporine is safe and efficacious in de novo renal transplant recipients treated with enteric‐coated mycophenolic acid and basiliximab [abstract no: F‐PO1088]. Journal of the American Society of Nephrology 2006;17(Abstracts):565A. [CENTRAL: CN‐00644166] - PubMed
-
- Budde K, Zeier M, Cohen D, Kirchherr B, MyProms Study Group. How much exposure is needed in the first week in patients receiving induction with basiliximab and enteric coated mycophenolate sodium? [abstract no: 1206]. American Journal of Transplantation 2005;5(Suppl 11):464. [CENTRAL: CN‐00602063]
CAESAR Study 2007 {published data only}
-
- Ekberg H, Grinyo J, Nashan B, Vanrenterghem Y, Vincenti F, CAESAR Study Group. Low‐dose cyclosporine in conjunction with daclizumab, mycophenolate mofetil and corticosteroids is safe and effective in contrast to early cyclosporine withdrawal [abstract]. 3rd International Congress on Immunosuppression; 2004 Dec 8‐11; San Diego (CA). 2004. [CENTRAL: CN‐00550672]
-
- Ekberg H, Grinyo J, Nashan B, Vanrenterghem Y, Vincenti F, Calleja E, et al. The use of daclizumab and mycophenolate mofetil in combination with corticosteroids and cyclosporine (low dose versus low dose followed by withdrawal) to optimize renal function in recipients of renal allografts [abstract]. Transplantation 2004;78(2 Suppl):458. [CENTRAL: CN‐00509171]
-
- Ekberg H, Grinyo J, Nashan B, Vanrenterghem Y, Vincenti F, Voulgari A, et al. Cyclosporine sparing with mycophenolate mofetil, daclizumab and corticosteroids in renal allograft recipients: the CAESAR Study. American Journal of Transplantation 2007;7(3):560‐70. [MEDLINE: ] - PubMed
-
- Grinyo J, Vanrenterghem Y, Nashan B, Vincenti F, Ekberg H, Spleiss O, et al. Association of three polymorphisms with acute rejection after kidney transplantation: an exploratory pharmacogenetic analysis of a randomized multicenter clinical trial (the CAESAR study) [abstract no: 1020]. American Journal of Transplantation 2006;6(Suppl 2):410. [CENTRAL: CN‐00678972]
-
- Kuypers DR, Ekberg H, Grinyo J, Nashan B, Vincenti F, Snell P, et al. Mycophenolic acid exposure after administration of mycophenolate mofetil in the presence and absence of cyclosporin in renal transplant recipients. Clinical Pharmacokinetics 2009;48(5):329‐41. [MEDLINE: ] - PubMed
Cai 2014 {published data only}
-
- Cai L, Zeng F, Liu B, Wei L, Chen Z, Jiang J. A single‐centre, open‐label, prospective study of an initially short‐term intensified dosing regimen of enteric‐coated mycophenolate sodium with reduced cyclosporine A exposure in Chinese live‐donor kidney transplant recipients. International Journal of Clinical Practice. Supplement 2014;68(181):23‐30. [MEDLINE: ] - PubMed
CALFREE Study 2010 {published data only}
-
- Franz S, Regeniter A, Hopfer H, Mihatsch M, Dickenmann M. Tubular toxicity in sirolimus‐ and cyclosporine‐based transplant immunosuppression strategies: an ancillary study from a randomized controlled trial. American Journal of Kidney Diseases 2010;55(2):335‐43. [MEDLINE: ] - PubMed
-
- Giannini O, Dickenmann M, Kim MJ, Franz S, Mayr M, Mihatsch MJ, et al. The CALFREE Study ‐ an open, prospective, randomized single center study to investigate calcineurin free immunosuppression in 100 de novo, normal risk renal transplant recipients: preliminary results [abstract no: P04.02]. Kidney & Blood Pressure Research 2004;27(5‐6):329. [CENTRAL: CN‐00615861]
CENTRAL Study 2012 {published data only}
-
- Mjornstedt L, Schwartz Sorensen S, Zur Muhlen B, Jespersen B, Hansen JM, Bistrup C, et al. Renal function three years after early conversion from a calcineurin inhibitor to everolimus: results from a randomized trial in kidney transplantation. Transplant International 2015;28(1):42‐51. [MEDLINE: ] - PubMed
-
- Mjornstedt L, Sorensen SS, Zur Muhlen B, Jespersen B, Hansen JM, Bistrup C, et al. Improved renal function after early conversion from a calcineurin inhibitor to everolimus: a randomized trial in kidney transplantation. American Journal of Transplantation 2012;12(10):2744‐53. [MEDLINE: ] - PubMed
-
- Murbraech K, Holdaas H, Massey R, Undset LH, Aakhus S. Cardiac response to early conversion from calcineurin inhibitor to everolimus in renal transplant recipients: An echocardiographic substudy of the randomized controlled CENTRAL trial. Transplantation 2014;97(2):184‐7. [MEDLINE: ] - PubMed
-
- Murbraech K, Massey R, Undset LH, Midtvedt K, Holdaas H, Aakhus S. Cardiac response to early conversion from calcineurin inhibitor to everolimus in renal transplant recipients ‐ a three‐yr serial echocardiographic substudy of the randomized controlled CENTRAL trial. Clinical Transplantation 2015;29(8):678‐84. [MEDLINE: ] - PubMed
CERTITEM Study 2015 {published data only}
-
- Hertig A, Kamar N, Albano L, Anglicheau D, Durrbach A, Vuiblet V, et al. Epithelial to mesenchymal transition markers in kidney transplant recipients: The CERTITEM trial [abstract]. Transplantation 2014;98:81. [EMBASE: 71543793]
-
- Hertig A, Kamar N, Anglicheau D, Moulin B, Hazzan M, Hurault De Ligny B, et al. Epithelial to mesenchymal transition markers in kidney transplant recipients: The CERTITEM trial [abstract no: O5]. Transplant International 2013;26(Suppl 3):2. [EMBASE: 71356080]
-
- Hertig A, Kamar N, Anglicheau D, Moulin B, Hazzan M, Ligny BH, et al. Epithelial to mesenchymal transition markers in kidney transplant recipients: The CERTITEM trial [abstract no: P081]. Transplant International 2013;26(Suppl 2):201. [EMBASE: 71359712]
-
- Rostaing L, Hertig A, Albano L, Anglicheau D, Durrbach A, Vuiblet V, et al. Fibrosis progression according to epithelial‐mesenchymal transition profile: a randomized trial of everolimus versus CsA. American Journal of Transplantation 2015;15(5):1303‐12. [MEDLINE: ] - PubMed
Chadban 2013 {published data only}
-
- Chadban S, Campbell S, Russ G, Walker R, Chapman J, Pussell B, et al. A one‐year, randomised, open label, parallel group study to investigate the safety and efficacy of enteric‐coated mycophenolate sodium (EC‐MPS) in combination with full dose or reduced dose cyclosporine microemulsion (CSA‐ME), basiliximab and steroids in de novo kidney transplantation [abstract no: 32]. 24th Annual Scientific Meeting. Transplantation Society of Australia & New Zealand (TSANZ); 2006 Mar 29‐31; Canberra, Australia. 2006:51. [CENTRAL: CN‐00583470]
-
- Chadban S, Eris J, Russ G, Campbell S, Chapman J, Pussell B, et al. Enteric‐coated mycophenolate sodium in combination with full dose or reduced dose cyclosporine, basiliximab and corticosteroids in Australian de novo kidney transplant patients. Nephrology 2013;18(1):63‐70. [MEDLINE: ] - PubMed
Chan 2008 {published data only}
-
- Chan L, Greenstein S, Hardy MA, Hartmann E, Bunnapradist S, Cibrik D, et al. Multicenter, randomized study of the use of everolimus with tacrolimus after renal transplantation demonstrates its effectiveness. Transplantation 2008;85(6):821‐6. [MEDLINE: ] - PubMed
-
- Chan L, Hartmann E, Cibrik D, Cooper M, Shaw LM. Everolimus (RAD001) concentration is associated with risk reduction for acute rejection in de novo renal transplant recipients [abstract no: SA‐PO2529]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):678A. [CENTRAL: CN‐00716072]
-
- Chan L, Hartmann E, Cibrik D, Cooper M, Shaw LM. Optimal everolimus concentration is associated with risk reduction for acute rejection in de novo renal transplant recipients. Transplantation 2010;90(1):31‐7. [MEDLINE: ] - PubMed
Chan 2012 {published data only}
-
- Asderakis A, Chan L. Renal function with enteric‐coated mycophenolate sodium in combination with reduced and standard tacrolimus levels: results of a 6‐month study in de novo renal transplant recipients [abstract no: O111]. British Transplantation Society (BTS).12th Annual Congress; 2009 Apr 21‐24; Liverpool, UK. 2009.
-
- Chan L, Hart M, Andres A, Gugliuzza K, Bunnapradist S, Parasumaran R. Renal function and incidence of NODM with enteric‐coated mycophenolate sodium in combination with reduced and standard tacrolimus levels: results of a 6‐month comparative study in de novo renal transplant recipients [abstract no: 718]. Transplantation 2008;86(2S):251.
-
- Nicholson ML, Chan L. Incidence of new onset diabetes mellitus in de novo renal transplant recipients treated with enteric‐coated mycophenolate sodium in combination with reduced or standard tacrolimus target levels: results of a 6‐month randomized study [abstract no: P266]. British Transplantation Society (BTS).12th Annual Congress; 2009 Apr 21‐24; Liverpool, UK. 2009.
Chhabra 2013 {published data only}
-
- Alvarado A, Chhabra D, Wang E, Najafian N, Friedewald J, Ho B, et al. Prospective randomized study to evaluate the feasability of CNI elimination with conversion to sirolimus in prednisone‐free immunosuppressive regimen [abstract no: 53]. American Journal of Transplantation 2012;12(Suppl S3):42. [EMBASE: 70746000]
-
- Alvarado A, Shetty A, Traitanon O, Leventhal J, Mas V, Chhabra D, et al. Calcineurin‐inhibitor conversion to mTOR inhibitor in renal transplant recipients leads to worse long term clinical outcomes [abstract]. American Journal of Transplantation 2015;15(Suppl 3). [EMBASE: 71953109]
-
- Chhabra D, Alvarado A, Dalal P, Leventhal J, Wang C, Sustento‐Reodica N, et al. Impact of calcineurin‐inhibitor conversion to mTOR inhibitor on renal allograft function in a prednisone‐free regimen. American Journal of Transplantation 2013;13(11):2902‐11. [MEDLINE: ] - PubMed
-
- Shah G, Xu L, Dalal P, Chhabran D, Friedewald J, Ho B, et al. Conversion from CNI to SRL in a pred‐free immunosuppressive regimen: interim report of a prospective randomized study [abstract no: 1641]. American Journal of Transplantation 2010;10(Suppl 4):504.
Cibrik 2007 {published data only}
-
- Bresnahan B, Cibrik D, Jensik S, Whelchel J, Klintmalm G, Cohen D, et al. Treatment of high‐risk renal transplant recipients with EC‐MPS (Myfortic®) is safe and efficacious [abstract no: PUB216]. Journal of the American Society of Nephrology 2005;16:829A. [CENTRAL: CN‐00644277]
-
- Budde K, Zeier M, Cohen D, Kirchherr B, MyProms Study Group. How much exposure is needed in the first week in patients receiving induction with basiliximab and enteric coated mycophenolate sodium? [abstract no: 1206]. American Journal of Transplantation 2005;5(Suppl 11):464. [CENTRAL: CN‐00602063]
-
- Cibrik D, Jensik S, Bresnahan B, Whelchel J, Klintmalm G, ERL2405‐US01 Study Group. Safety and efficacy of EC‐MPS in combination with simulect and neoral in de novo renal transplant high‐risk recipients [abstract no: 135]. American Journal of Transplantation 2005;5(Suppl 11):190. [CENTRAL: CN‐00644170]
-
- Cibrik D, Jensik S, Meier‐Kriesche H, Bresnahan B, Lieberman B, Myfortic US01 Renal Transplant Group. Enteric‐coated mycophenolate sodium in combination with optimized neoral dosing, basiliximab, and steroids results in good efficacy and renal function in renal transplant recipients in the first six months [abstract no: 220]. American Journal of Transplantation 2004;4(Suppl 8):218. [CENTRAL: CN‐00644278]
-
- Cibrik D, Meier‐Kriesche HU, Bresnahan B, Wu YM, Klintmalm G, Kew CE, et al. Renal function with cyclosporine C2 monitoring, enteric‐coated mycophenolate sodium and basiliximab: a 12‐month randomized trial in renal transplant recipients. Clinical Transplantation 2007;21(2):192‐201. [MEDLINE: ] - PubMed
Cockfield 2002 {published data only}
-
- Cockfield S, Whelchel J, MacDonald A. An open‐label, concentration‐controlled, randomized, 6‐month study of standard‐dose tacrolimus + sirolimus + corticosteroids compared to reduced‐dose tacrolimus + sirolimus + corticosteroids in renal allograft recipients [abstract no: 1021]. American Journal of Transplantation 2002;2(Suppl 3):395. [MEDLINE: ]
-
- Daloze P, Whelchel J, Cockfield S, MacDonald A. A 6‐month multicenter randomized concentration‐controlled study of reduced‐dose tacrolimus (TAC)+sirolimus(SRL)+corticosteroids(CS) compared to standard‐dose TAC+SRL+CS in clinical kidney transplantation [abstract no: 0555]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00415490]
CONCEPT Study 2009 {published data only}
-
- Chun DX, Alexandre H, Sandrine GS, Olivier T, Isabelle E, Christophe L, et al. The phenotype of tubular epithelial cells does not recover after a conversion from cyclosporine A to siroliumus [abstract]. Nephrology Dialysis Transplantation 2012;27(Suppl 2):ii517. [EMBASE: 70766851]
-
- Joannides R, Monteil C, Ligny BH, Westeel PF, Iacob M, Thervet E, et al. Immunosuppressant regimen based on sirolimus decreases aortic stiffness in renal transplant recipients in comparison to cyclosporine. American Journal of Transplantation 2011;11(11):2414‐22. [MEDLINE: ] - PubMed
-
- Lebranchu Y, Etienne I, Touchard G, Thervet E, Westell P, Toupance O, et al. Comparison of efficacy and safety of cyclosporine (CsA) discontinuation with introduction of sirolimus (SRL) at week 12 to standard strategy in renal transplant recipients receiving mycophenolate mofetil (MMF) [abstract no: 53]. American Journal of Transplantation 2007;7(Suppl 2):160. [CENTRAL: CN‐00724873]
-
- Lebranchu Y, Thierry A, Thervet E, Buchler M, Etienne I, Westeel PF, et al. Efficacy and safety of early cyclosporine conversion to sirolimus with continued MMF‐four‐year results of the Postconcept study. American Journal of Transplantation 2011;11(8):1665‐75. [MEDLINE: ] - PubMed
-
- Lebranchu Y, Thierry A, Toupance O, Westeel PF, Etienne I, Thervet E, et al. Efficacy on renal function of early conversion from cyclosporine to sirolimus 3 months after renal transplantation: Concept study. American Journal of Transplantation 2009;9(5):1115‐23. [MEDLINE: ] - PubMed
CONVERT Trial 2009 {published data only}
-
- Alberu J, Pascoe MD, Campistol JM, Schena FP, Rial Mdel C, Polinsky M, et al. Lower malignancy rates in renal allograft recipients converted to sirolimus‐based, calcineurin inhibitor‐free immunotherapy: 24‐month results from the CONVERT trial. Transplantation 2011;92(3):303‐10. [MEDLINE: ] - PubMed
-
- Alberu J, Schena FP, Wali R, Pascoe M, Carmen Rial M, The Sirolimus CONVERT Trial Study Group. Lower malignancy rates in renal allograft recipients converted to sirolimus (SRL)‐based, calcineurin inhibitor‐free immunotherapy: 24 month results from the CONVERT trial [abstract no: TH‐FC082]. Journal of the American Society of Nephrology 2006;17(Abstracts):18A. [CENTRAL: CN‐00601954]
-
- Brennan D, Schena FP, Wali R, The Sirolimus CONVERT Trial Study Group. Factors contributing to the development of proteinuria after conversion from calcineurin inhibitors to sirolimus: results from the multicenter CONVERT trial [abstract no: TH‐FC083]. Journal of the American Society of Nephrology 2006;17(Abstracts):18A. [CENTRAL: CN‐00601955]
-
- Chapman J, Campistol JM, Grinyo JM, Morales JM, Mota A, Schena FP. Results from a long‐term extension study in renal allograft recipients show that sirolimus‐based therapy without cyclosporine is a safe alternative to sirolimus plus cyclosporine therapy. [abstract no: F‐PO1050]. Journal of the American Society of Nephrology 2004;15(Oct):295A. [CENTRAL: CN‐00583164]
-
- Oberbauer R, Schena FP, Wali R, Pascoe M, Alberu J, Carmen Rial M, et al. Conversion from calcineurin inhibitors to sirolimus compared with continued use of calcineurin inhibitors in renal allograft recipients: 24‐month safety and efficacy results from the CONVERT trial [abstract no: F‐FC154]. Journal of the American Society of Nephrology 2006;17(Abstracts):69A. [CENTRAL: CN‐00601952]
CTOT‐09 Study 2015 {published data only}
-
- Formica R, Nickerson P, Poggio E, Tinckam K, Rush D, Gibson I, et al. Immune monitoring and tacrolimus (TAC) withdrawal in low risk recipients of kidney transplants‐results of CTOT09 [abstract]. Transplantation 2014;98(Suppl 1):225. [EMBASE: 71544207]
-
- Nickerson P, Wiebe C, Formica R, Tinckam K, Poggio E, Bunnapradist S, et al. Epitope mismatch predicts de novo DSA after CNI withdrawal in low‐risk kidney transplant recipients [abstract]. Transplantation 2014;98(Suppl 1):137. [EMBASE: 71543963]
de Sevaux 2001 {published data only}
-
- Smak Gregoor PJ, Sevaux RG, Hene RJ, Hilbrands LB, Gelder T, Hoitsma AJ, et al. The cyclosporine sparing effect of mycophenolate mofetil, a randomized study [abstract]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):699A. [CENTRAL: CN‐00447776]
-
- Sevaux RG, Gregoor PJ, Hene RJ, Hoitsma AJ, Vos P, Weimar W, et al. A controlled trial comparing two doses of cyclosporine in conjunction with mycophenolate mofetil and corticosteroids. Journal of the American Society of Nephrology 2001;12(8):1750‐7. [MEDLINE: ] - PubMed
-
- Sevaux RG, Smak Gregoor PJ, Hene RJ, Hoitsma AJ, Vos P, et al. Addition of mycophenolate mofetil to cyclosporine and prednisone allows for a lower dose of cyclosporine after renal transplantation [abstract]. XVIII International Congress of the Transplantation Society; 2000 Aug 27‐Sept 1; Rome, Italy. 2000. [CENTRAL: CN‐00583239]
-
- Sévaux RG, Smak Gregoor PJ, Hené RJ, Weimar W, Hoitsma AJ, Gelder T, et al. A randomised study of conventional vs low dose cyclosporine in renal transplant recipients treated with cyclosporine, mycophenolate mofetil and prednisone [abstract no: 418]. Transplantation 1998;65(12):S107. [CENTRAL: CN‐00583548]
DICAM Study 2010 {published data only}
-
- Etienne I, Toupance O, Benichou J, Thierry A, Al Najjar A, Hurault dL, et al. A 50% reduction in cyclosporine exposure in stable renal transplant recipients: renal function benefits. Nephrology Dialysis Transplantation 2010;25(9):3096‐106. [MEDLINE: ] - PubMed
Dudley 2005 {published data only}
-
- Dudley C, Pohanka E, Riad H, Dedochova J, Wijngaard P, Marshall S, et al. MMF substitution for CSA in chronic allograft dysfunction: 2 year follow‐up of a multi‐center randomized controlled study [abstract no: 1513]. American Journal of Transplantation 2005;5(Suppl 11):541.
-
- Dudley C, Pohanka E, Riad H, Dedochova J, Wijngaard P, Sutter C, et al. Mycophenolate mofetil substitution for cyclosporine a in renal transplant recipients with chronic progressive allograft dysfunction: the "creeping creatinine" study. Transplantation 2005;79(4):466‐75. [MEDLINE: ] - PubMed
-
- Dudley CR, MMF 'Creeping Creatinine' Study Group. MMF substitution for CSA is an effective and safe treatment of chronic allograft dysfunction; results of a multi‐center randomized controlled study [abstract no: 41]. American Journal of Transplantation 2002;2(Suppl 3):148.
-
- Tedesco de Silva H. MMF replacing CSA reverses the "creeping creatinine" of renal transplant recipients of a multi‐center randomized controlled study [abstract]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00416751]
El‐Agroudy 2014 {published data only}
-
- El‐Agroudy A, Alarrayed S, Ghareeb S, Farid E, Alhellow H, Abdulla S. Long‐term outcome of a prospective randomized trial of conversion from tacrolimus to sirolimus treatment after renal transplantation [abstract no: B964]. Transplantation 2014;98(Suppl 1):539. [EMBASE: 71545346]
Fangmann 2010 {published data only}
-
- Fangmann J, Arns W, Marti H, Budde K, Beckurts T, Hauss J. Low dose cyclosporine regimen with daclizumab induction and mycophenolate mofetil after kidney transplantation ‐ impact on renal function and rejection episodes [abstract no: 113]. American Journal of Transplantation 2005;5(Suppl 11):185. [CENTRAL: CN‐00644197]
-
- Fangmann J, Arns W, Marti H, Budde K, Neumayer H, Beckurts T, et al. Impact of daclizumab and low dose cyclosporine in combination with mycophenolate mofetil and steroids on renal function after kidney transplantation [abstract no: 715]. American Journal of Transplantation 2004;4(Suppl 8):353. [CENTRAL: CN‐00509182]
-
- Fangmann J, Arns W, Marti H, Budde K, Neumayer H, Beckurts T, et al. Impact of daclizumab and low dose cyclosporine in combination with mycophenolate mofetil and steroids on renal function after kidney transplantation [abstract no: P249]. Transplantation 2004;78(2 Suppl):280.
-
- Fangmann J, Arns W, Marti HP, Hauss J, Ketteler M, Beckurts T, et al. Impact of daclizumab, low‐dose cyclosporine, mycophenolate mofetil and steroids on renal function after kidney transplantation. Nephrology Dialysis Transplantation 2010;25(1):283‐92. [MEDLINE: ] - PubMed
Ferguson 2006 {published data only}
-
- Ferguson R, Mulgaonkar S, Tedesco Silva H, Oppenheimer F, Walker R, et al. FTY720 with reduced‐exposure (R‐E) cyclosporine [neoral] (CsA): acute rejection prophylaxis in de novo renal transplant (Tx) recipients [abstract]. Transplantation Society of Australia and New Zealand. 21st Annual Meeting; 2003 Apr 9‐11; Canberra (ACT). 2003:63. [CENTRAL: CN‐00445317]
-
- Ferguson RM, Mulgaonkar S, Tedesco H, Oppenheimer F, Walker R, Kunzendor U, et al. FTY720 with reduced‐exposure neoral provides adequate rejection prophylaxis in de novo renal transplant recipients. Interim results [abstract no: 0049]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00415638]
-
- Ferguson RM, Mulgaonkar S, Tedesco H, Oppenheimer F, Walker R, Russ G, et al. High efficacy of FTY720 with reduced cyclosporine dose in preventing rejection in renal transplantation: 12‐month preliminary results [abstract no: 624]. American Journal of Transplantation 2003;3(Suppl 5):311. [CENTRAL: CN‐00445316]
-
- Mulgaonkar S, Tedesco H, Oppenheimer F, Walker R, Kunzendorf U, Russ G, et al. FTY720/cyclosporine regimens in de novo renal transplantation: a 1‐year dose‐finding study.[Erratum appears in Am J Transplant. 2006 Dec;6(12):3044]. American Journal of Transplantation 2006;6(8):1848‐57. [MEDLINE: ] - PubMed
-
- Russ G, Ferguson RM, Mulgaonkar S, Tedesco‐Silva H, Oppenheimer F, Walker R, et al. FTY720 with reduced dose Neoral (RDN) may offer a safety/tolerability profile advantage over conventional immunosuppressive therapies [abstract no: 1000]. American Journal of Transplantation 2004;4(Suppl 8):432. [CENTRAL: CN‐00509451]
Flechner‐318 Study 2002 {published data only}
-
- Flechner SM, Burke JT, Cook DJ, Mastroianni B, Savas K, Goldfarb D, et al. A randomized prospective trial of sirolimus vs cyclosporine in kidney transplantation: renal function and histology at two years [abstract]. American Journal of Transplantation 2003;3(Suppl 5):450. [CENTRAL: CN‐00445351]
-
- Flechner SM, Cook DJ, Goldfarb D, Modlin C, Mastroianni B, Savas K, et al. A randomized trial of sirolimus vs cyclosporine in kidney transplantation: impact on blood cells, lymphocyte subsets, and flow crossmatches [abstract no: 1317]. American Journal of Transplantation 2002;2(Suppl 3):470.
-
- Flechner SM, Cook DJ, Goldfarb D, Modlin C, Mastroianni B, Savas K, et al. A randomized trial of sirolimus vs cyclosporine in kidney transplantation: impact on blood cells, lymphocyte subsets, and flow crossmatches [abstract]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00415657]
-
- Flechner SM, Goldfarb D, Modlin C, Feng J, Krishnamurthi V, Mastroianni B, et al. Kidney transplantation without calcineurin inhibitor drugs: a prospective, randomized trial of sirolimus versus cyclosporine. Transplantation 2002;74(8):1070‐6. [MEDLINE: ] - PubMed
-
- Flechner SM, Goldfarb D, Solez K, Modlin CS, Mastroianni B, Savas K, et al. Kidney transplantation with sirolimus and mycophenolate mofetil‐based immunosuppression: 5‐year results of a randomized prospective trial compared to calcineurin inhibitor drugs. Transplantation 2007;83(7):883‐92. [MEDLINE: ] - PubMed
Garcia 2007 {published data only}
-
- Garcia R, Hanzawa NM, Machado PG, Moreira SR, Prismich G, Felipe CR, et al. A calcineurin inhibitor‐free regimen for low risk kidney transplant recipients [abstract no: 2379]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00520337]
-
- Garcia R, Machado PG, Felipe CR, Park SI, Spinelli GA, Franco MF, et al. Exploratory calcineurin inhibitor‐free regimens in living‐related kidney transplant recipients. Brazilian Journal of Medical & Biological Research 2007;40(4):457‐65. [MEDLINE: ] - PubMed
Grimbert 2002 {published data only}
-
- Grimbert P, Baron C, Fruchaud G, Hemery F, Desvaux D, Buisson C, et al. Long‐term results of a prospective randomized study comparing two immunosuppressive regimens, one with and one without CsA, in low‐risk renal transplant recipients. Transplant International 2002;15(11):550‐5. [MEDLINE: ] - PubMed
Grinyo 2004 {published data only}
-
- Campistol JM, Grinyo JM, Paul J, Garcia J, Arias M, Morales JM, et al. Sirolimus, TAC and corticosteroids in the postoperative period. Preliminary results [abstract]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002.
-
- Grinyo JM, Campistol JM, Paul J, Garcia J, Arias M, Morales JM, et al. A randomised, open, multicenter, trial comparing tacrolimus (TAC) withdrawal with TAC dose reduction in de novo renal transplants, receiving sirolimus (SIR), TAC and steroids in the postoperative time. Initial results [abstract no: SA‐PO0491]. Journal of the American Society of Nephrology 2002;13(September, Program & Abstracts):363A. [CENTRAL: CN‐00445561]
-
- Grinyo JM, Campistol JM, Paul J, Garcia J, Arias M, Morales JM, et al. A randomised, open‐label, pilot study to compare the safety and efficacy of tacrolimus (TAC) elimination with tac dose reduction in de novo renal allograft recipients, receiving sirolimus, tac and corticosteroids in the postoperative period. Preliminary results [abstract]. American Journal of Transplantation 2002;2(Suppl 3):394.
-
- Grinyo JM, Campistol JM, Paul J, Garcia‐Martinez J, Morales JM, Prats D, et al. Pilot randomized study of early tacrolimus withdrawal from a regimen with sirolimus plus tacrolimus in kidney transplantation. American Journal of Transplantation 2004;4(8):1308‐14. [MEDLINE: ] - PubMed
-
- Morales JM, Grinyo JM, Campistol JM, Garcia J, Arias M, Paul J, et al. FK‐506 withdrawal from a regimen of sirolimus plus FK‐506 results at 2 years in an improvement in renal function, blood pressure and proteinuria in ongoing kidney recipients [abstract no: 1192]. American Journal of Transplantation 2005;5(Suppl 11):460. [CENTRAL: CN‐00724880]
Hall 1988 {published data only}
-
- Gallagher M, Jardine M, Cass A, Perkovic V, Petrie J, McDonald S, et al. 20 year outcomes of the Australian multicentre randomised trial of cyclosporine withdrawal in renal transplantation [abstract no: 1075]. Nephrology 2007;12(Suppl 2):A19. [CENTRAL: CN‐00653709]
-
- Gallagher M, Jardine M, Cass A, Perkovic V, Petrie J, McDonald S, et al. 20 year outcomes of the Australian multicentre randomised trial of cyclosporine withdrawal in renal transplantation [abstract]. Immunology & Cell Biology 2007;85(4):A19.
-
- Gallagher M, Jardine M, Perkovic V, Cass A, McDonald S, Petrie J, et al. Cyclosporine withdrawal improves long‐term graft survival in renal transplantation. Transplantation 2009;87(12):1877‐83. [MEDLINE: ] - PubMed
-
- Gallagher M, Webster A, Jardine M, Perkovic V, Cass A, Eris J. Twenty year cancer outcomes of a randomized trial of immunosuppression in kidney transplant recipients: results of the Australian multicentre trial of cyclosporine withdrawal [abstract no: 078]. Nephrology 2008;13(Suppl 3):A119. [CENTRAL: CN‐00758532]
-
- Gallagher MP, Eris JM, Tiller DJ, Hall BM. Long term outcome of Australian multicentre trial of cyclosporin withdrawal in cadaveric renal transplantation [abstract]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00415694]
Hazzan 2005 {published data only}
-
- Hazzan M, Buob D, Labalette M, Provot F, Glowacki F, Hoffmann M, et al. Assessment of the risk of chronic allograft dysfunction after renal transplantation in a randomized cyclosporine withdrawal trial. Transplantation 2006;82(5):657‐62. [MEDLINE: ] - PubMed
-
- Hazzan M, Copin MC, Labalette M, Glowacki F, Provot F, Roumilhac D, et al. Predictive factors of acute rejection after early cyclosporine withdrawal in renal transplant recipients receiving mycophenolate mofetil: results from a prospective, randomized trial [abstract]. American Journal of Transplantation 2003;3(Suppl 5):197. [CENTRAL: CN‐00445673] - PubMed
-
- Hazzan M, Hertig A, Buob D, Noel C, Copin M, Rondeau E, et al. Cyclosporin induces epithelial to mesenchymal transition in renal grafts [abstract no: 131]. American Journal of Transplantation 2008;8(Suppl 2):214.
-
- Hazzan M, Labalette M, Copin MC, Glowacki F, Provot F, Pruv FR, et al. Predictive factors of acute rejection after early cyclosporine withdrawal in renal transplant recipients who receive mycophenolate mofetil: results from a prospective, randomized trial. Journal of the American Society of Nephrology 2005;16(8):2509‐16. [MEDLINE: ] - PubMed
Heering 1993 {published data only}
-
- Heering P, Westhoff A, Ivens K K, Grabensee B. Comparison between the effects of CSA and azathioprine on functional renal parameters in randomized study after renal transplantation [abstract]. Journal of the American Society of Nephrology 1993;4(Program & Abstracts):940. [CENTRAL: CN‐00484304]
-
- Heering P, Westhoff A, Ivens K, Helmchen U, Grabensee B. Increased risk in conversion of stable renal allografts from cyclosporin to azathioprine: a randomized controlled study [abstract]. 12th International Congress of Nephrology; 1993 Jun 13‐18; Jerusalem, Israel. 1993:194.
-
- Heering P, Westhoff K, Ivens B, Kutkuhn B, Grabensee H. Increased risk in conversion of stable renal allografts from cyclosporin to azathioprine [abstract]. Nephrology Dialysis Transplantation 1993;8(9):1041. [CENTRAL: CN‐00260889]
-
- Ivens K, Heering P, Withold W, Koch M, Reinauer H, Grabensee B. Withdrawal of cyclosporine improves the cardio‐vascular risk profile in renal graft recipients [abstract]. Journal of the American Society of Nephrology 1994;5(3):1013.
HERAKLES Study 2012 {published data only}
-
- Arns W, Budde K, Sommerer C, Witzke O, Guba M, Jacobi J, et al. Month 48 follow‐up results of HERAKLES trial on three different treatment regimen and switching off behaviour in de novo renal transplant patients [abstract]. American Journal of Transplantation 2015;15(Suppl 3). [EMBASE: 71953873]
-
- Arns W, Neumayer H, Lehner F, Witzke O, Sommerer C, Kliem V, et al. HERAKLES at month 24: follow‐up results on efficacy and safety of three different treatment regimens in de novo renal transplant patients demonstrate options for individualized immunosuppression [abstract no: BO148]. Transplant International 2013;26(Suppl 2):21. [EMBASE: 71356261]
-
- Arns W, Sommerer C, Witzke O, Lehner F, Zeier M, Neumayer HH, et al. Efficacy and safety of three different treatment regimens in de novo renal transplant patients: results of the HERAKLES trial [abstract no: 1722]. Transplantation 2012;94(10):995. [EMBASE: 71251769]
-
- Budde K, Arns W, Sommerer C, Lehner F, Zeier M, Neumayer H, et al. Superior renal function in an everolimus‐based calcineurin inhibitor free regimen compared to standard cyclosporine/ mycophenolate and low cyclosporine/everolimus: follow‐up of the HERAKLES study at month 36 [abstract no: 716]. Transplantation 2014;98(Suppl 1):81. [EMBASE: 71543792]
-
- Budde K, Arns W, Sommerer C, Lehner F, Zeier M, Neumayer H, et al. Superior renal function in an everolimus‐based calcineurin inhibitor free regimen compared to standard cyclosporine/mycophenolate and low cyclosporine/everolimus: follow‐up of the HERAKLES study at month 24 [abstract no: B932]. American Journal of Transplantation 2013;13(Suppl 5):310‐1. [EMBASE: 71057508]
Hollander 1995 {published data only}
-
- Bakker RC, Hollander AA, Mallat MJ, Bruijn JA, Paul LC, Fijter JW. Conversion from cyclosporine to azathioprine at three months reduces the incidence of chronic allograft nephropathy. Kidney International 2003;64(3):1027‐34. [MEDLINE: ] - PubMed
-
- Hollander AA, Saase JL, Kootte AM, Dorp WT, Bockel HJ, Es LA, et al. Beneficial effects of conversion from cyclosporin to azathioprine after kidney transplantation. Lancet 1995;345(8950):610‐4. [MEDLINE: ] - PubMed
Holm 2008 {published data only}
-
- Holm A, Hernandez M, Camarena A, Perez L, Santos M, Porras MA. Efficacy, tolerability and safety of mycophenolate mofetil (Cellcept) + sirolimus (Rapamune) as maintenance therapy after calcineurin inhibitor withdrawal in LRD and CAD, adults and pediatric renal transplant recipients (experience with 405 patients) [abstract no: 627]. Transplantation 2008;86(Suppl 2):220. [CENTRAL: CN‐00740576]
Isoniemi 1990 {published data only}
-
- Isoniemi H. Renal allograft immunosuppression V: glucose intolerance occurring in different immunosuppressive treatments. Clinical Transplantation 1991;5(3):268‐72. [EMBASE: 21188067]
-
- Isoniemi H. Renal allograft immunosuppression. III. Triple therapy versus three different combinations of double drug treatment: two year results in kidney transplant patients. Transplant International 1991;4(1):31‐7. [MEDLINE: ] - PubMed
-
- Isoniemi H, Ahonen J, Eklund B, Hockerstedt K, Salmela K, Willebrand E, et al. Renal allograft immunosuppression. II. A randomized trial of withdrawal of one drug in triple drug immunosuppression. Transplant International 1990;3(3):121‐7. [MEDLINE: ] - PubMed
-
- Isoniemi H, Ahonen J, Krogerus L, Eklund B, Hockerstedt K, Salmela K, et al. Chronic rejection of renal allografts with four immunosuppressive regimens. Transplantation Proceedings 1992;24(6):2716‐7. [MEDLINE: ] - PubMed
-
- Isoniemi H, Eklund B, Hockerstedt K, Korsback C, Salmela K, Willebrand E, et al. Discontinuation of one drug in triple drug treatment of renal allograft patients: 1‐year results. Transplantation Proceedings 1990;22(4):1365‐6. [MEDLINE: ] - PubMed
Kosch 2003a {published data only}
-
- Hausberg M, Lang D, Levers A, Suwelack B, Kisters K, Tokmak F, et al. Sympathetic nerve activity in renal transplant patients before and after withdrawal of cyclosporine. Journal of Hypertension 2006;24(5):957‐64. [MEDLINE: ] - PubMed
-
- Hausberg M, Suwelack B, Kosch M, Barenbrock M, Gerhardt U, Hohage H, et al. Effects of calcineurin‐inhibitor withdrawal on sympathetic nerve activity and blood pressure in renal transplant recipients [abstract]. Deutsche Medizinische Wochenschrift 2001;126:S193. [CENTRAL: CN‐00383083]
-
- Kosch M, Hausberg M, Suwelack B. Studies on effects of calcineurin inhibitor withdrawal on arterial distensibility and endothelial function in renal transplant recipients. Transplantation 2003;76(10):1516‐9. [MEDLINE: ] - PubMed
-
- Kosch M, Suwelack B, Barenbrock M, Kobelt V, Hohage H, Rahn KH, et al. Studies on effects of calcineurin‐inhibitor withdrawal on arterial distensibility and endothelial function in renal transplant recipients. Deutsche Medizinische Wochenschrift 2001;126:S185. [CENTRAL: CN‐00383367] - PubMed
Kreis 2003 {published data only}
-
- Kreis H, Miloradovich T, Mourad G, Cointault O, Berthoux F, Delahousse M, et al. Daclizumab and mycophenolate mofetil in renal transplant recipients: 2‐year outcome after early reduction of cyclosporine [abstract no: 1266]. American Journal of Transplantation 2003;3(Suppl 5):476. [CENTRAL: CN‐00446199]
-
- Kreis H, Miloradovich T, Mourad G, Cointault O, Berthoux F, Delahousse M, et al. Lowering cyclosporine dose is not associated with an increased risk of gastro‐intestinal adverse events nor the need for dosage decrease of mycophenolate mofetil [abstract no: P743]. Transplantation 2004;78(2 Suppl):462. [CENTRAL: CN‐00509292]
MacPhee 1998 {published data only}
-
- Joss N. Randomised study comparing cyclosporin with azathioprine one year after renal transplantation ‐ 15 year outcome [abstract]. Scottish Medical Journal 2005;50(2):82. [CENTRAL: CN‐00653805]
-
- Joss N, Glasgow Transplant Group. Randomised study comparing cyclosporin with azathioprine one year after renal transplantation ‐ 15 year outcome [abstract]. 41st Congress. European Renal Association. European Dialysis and Transplantation Association; 2004 May 15‐18; Lisbon, Portugal. 2004:197. [CENTRAL: CN‐00636135]
-
- Joss N, Rodger RS, McMillan MA, Junor BJ. Randomized study comparing cyclosporine with azathioprine one year after renal transplantation‐15‐year outcome data. Transplantation 2007;83(5):582‐7. [MEDLINE: ] - PubMed
-
- MacPhee IA, Bradley JA, Briggs JD, Junor BJ, Macpherson SG, McMillan MA, et al. Long‐term outcome of a prospective randomized trial of conversion from cyclosporine to azathioprine treatment one year after renal transplantation. Transplantation 1998;66(9):1186‐92. [MEDLINE: ] - PubMed
-
- MacPhee IA, Briggs JD, Junor BJ, McMillan MA, Rodger RS, Watson MA. A prospective randomised controlled trial of long‐term outcome for renal transplant recipients converted from cyclosporin to azathioprine treatment one year after transplantation [abstract]. Journal of the American Society of Nephrology 1996;7(9):1915. [CENTRAL: CN‐00602085]
Martinez‐Mier 2006 {published data only}
-
- Martinez‐Mier G, Mendez‐Lopez MT, Budar‐Fernandez LF, Estrada‐Oros J, Franco‐Abaroa R, George‐Micelli E, et al. Living related kidney transplantation without calcineurin inhibitors: Initial experience in a Mexican center. Transplantation 2006;82(11):1533‐6. [MEDLINE: ] - PubMed
MECANO Study 2009 {published data only}
-
- Baas MC, Gerdes VE, Berge IJ, Heutinck KM, Florquin S, Meijers JC, et al. Treatment with everolimus is associated with a procoagulant state. Thrombosis Research 2013;132(2):307‐11. [MEDLINE: ] - PubMed
-
- Baas MC, Kers J, Florquin S, Fijter JW, Heide JJ, Bergh Weerman MA, et al. Cyclosporine versus everolimus: effects on the glomerulus. Clinical Transplantation 2013;27(4):535‐40. [MEDLINE: ] - PubMed
-
- Baas MC, Struijk GH, Moes DJ, Berk IA, Jonkers RE, Fijter JW, et al. Interstitial pneumonitis caused by everolimus: a case‐cohort study in renal transplant recipients. Transplant International 2014;27(5):428‐36. [MEDLINE: ] - PubMed
-
- Bemelman FJ, Maar EF, Press RR, Kan HJ, Berge IJ, Homan van der Heide JJ, et al. Minimization of maintenance immunosuppression early after renal transplantation: an interim analysis. Transplantation 2009;88(3):421‐8. [MEDLINE: ] - PubMed
-
- Havenith SH, Yong SL, Donselaar‐van der Pant KA, Lier RA, Berge IJ, Bemelman FJ. Everolimus‐treated renal transplant recipients have a more robust CMV‐specific CD8+ T‐cell response compared with cyclosporine‐ or mycophenolate‐treated patients. Transplantation 2013;95(1):184‐91. [MEDLINE: ] - PubMed
MODIFY Study 2012 {published data only}
-
- David‐Neto E, Lemos F, Antonopoulos I, Souza P, Piovesan A, Ventura C, Kanashiro H, et al. Five‐year follow‐up of the MoDIFY study (modification of doses to improve function through the years) shows benefit of low tacrolimus and regular MMF doses [abstract]. Journal of Urology 2011;185(4 Suppl 1):e828. [EMBASE: 70378695]
-
- David‐Neto E, Lemos FC, Souza PS, Ventura CG, Castro MCR\, Agena F, et al. Five‐year follow‐up of the MoDIFY Study (Modification of Doses to Improve Function through the Years) shows benefit of low tacrolimus and regular MMF doses [abstract no: 1655]. American Journal of Transplantation 2010;10(Suppl 4):508. [EMBASE: 70465030]
-
- David‐Neto E, Pereira LM, Castro MC, Mattos RM, Sumita NM, Mendes E, et al. Interim analysis of the MODIFY study in renal transplantation [abstract]. Transplantation 2004;78(2 Suppl):278‐9. [CENTRAL: CN‐00509408]
-
- David‐Neto E, Pereira LM, Castro MC, Mattos RM, Sumita NM, Mendes E, et al. Therapeutic levels of mycophenolic acid under fixed doses of MMF on the first two months after kidney transplantation [abstract]. Transplantation 2004;78(2 Suppl):278.
-
- David‐Neto E, Pereira LM, Castro CR, Mattos RM, Sumita NM, Mendes ME, et al. Interim analysis of the MODIFY study in renal transplantation (Modification of Doses to Improve Function through the Years) [abstract]. American Journal of Transplantation 2004;4(Suppl 8):234‐5. [CENTRAL: CN‐00509150]
Muhlbacher 2014 {published data only}
-
- Muehlbacher F, Neumayer HH, Castillo D, Stefoni S, European Sirolimus CsA Minimisation Study Group. An open‐label study to evaluate the efficacy & safety of cyclosporine reduction in de novo renal allograft recipients receiving sirolimus: a dose comparative study [abstract no: 1224]. American Journal of Transplantation 2003;3(Suppl 5):465. [CENTRAL: CN‐00446852]
-
- Muhlbacher F, Neumayer HH, Castillo D, Stefoni S, Zygmunt AJ, Budde K, et al. The efficacy and safety of cyclosporine reduction in de novo renal allograft patients receiving sirolimus and corticosteroids: results from an open‐label comparative study. Transplant International 2014;27(2):176‐86. [MEDLINE: ] - PubMed
-
- Muhlbacher F, Paczek L. An open‐label study to evaluate the efficacy & safety of cyclosporine reduction in de novo renal allograft recipients receiving sirolimus: a dose comparative study [abstract no: 399]. American Journal of Transplantation 2002;2(Suppl 3):238.
-
- Neumayer H, Muhlbacher F, Stefoni S, Castillo D, Myfortic Maintenance Renal Transplantation Study Group. An open‐label study to evaluate the efficacy & safety of cyclosporine reduction in de novo renal allograft recipients receiving sirolimus: a dose comparative study [abstract]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00416320]
-
- Neumayer HH, Muehlbacher F, Castillo D, Stefoni S, European Sirolimus CsA Minimisation Study Group. An open‐label study to evaluate the efficacy & safety of cyclosporine reduction in de novo renal allograft recipients receiving sirolimus: a dose comparative study [abstract no: W738]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):785. [CENTRAL: CN‐00446926]
Nafar 2012 {published data only}
-
- Nafar M, Alipour B, Ahmadpoor P, Pour‐Reza‐Gholi F, Samadian F, Samavat S, et al. Sirolimus versus calcineurin inhibitor‐based immunosuppressive therapy in kidney transplantation: a 4‐year follow‐up. Iranian Journal of Kidney Diseases 2012;6(4):300‐6. [MEDLINE: ] - PubMed
Nashan 2004 {published data only}
-
- Curtis J, Nashan B, Poniicelli C, Mourad G, Boger R, RAD 156 Study Group. One year result of a multicenter, open‐label trial on safety and efficacy of certican (RAD) used in combination with simulect, corticosteroids, and full or reduced dose neoral in renal transplant [abstract no: 1335]. American Journal of Transplantation 2001;1(Suppl 1):474. [CENTRAL: CN‐00444958]
-
- Curtis J, Nashan B, Ponticelli C, Mourad G, Boger R, RAD 156 Study Group. Everolimus (RAD) in combination with reduced dose with reduced dose neoral® maintains effective immunosuppression with improved tolerability compared to full dose neoral [abstract no: A4625]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):884A. [CENTRAL: CN‐00550720]
-
- Mourad G, Boger R, Curtis J, Nashan B, Ponticelli C, RAD B 156 Study Group. CERTICAN (TM) (RAD; EVEROLIMUS), the proliferation signal inhibitor, is complementary with a reduced dose NEORAL® based quadruple immunosuppressive regimen [abstract no: 1622]. A Transplant Odyssey; 2001 Aug 20‐23; Istanbul, Turkey. 2001. [CENTRAL: CN‐00487738]
-
- Nashan B, Curtis J, Ponticelli C, Mourad G, Boger R, RAD B156 Study Group. Certican (RAD; everolimus), the proliferation signal inhibitor, is complementary with a reduced dose neoral based quadruple immunosuppressive regimen [abstract no: 398]. 10th ESOT & 12th ETCO Congress. Bridging the Future; 2001 Oct 6‐11; Lisboa, Portugal. 2001. [CENTRAL: CN‐00487741]
-
- Nashan B, Mourad G, Boger R, Curtis J, Ponticelli C, Haas T. Everolimus and reduced‐exposure cyclosporine in de novo renal‐transplant recipients: a three‐year phase II, randomized, multicenter, open‐label study. Transplantation 2004;78(9):1332‐40. [MEDLINE: ] - PubMed
Oh 2012 {published data only}
-
- Oh CK, Ha JW, Kim YH, Kim YL, Kim YS. Safety and efficacy of the early introduction of everolimus (Certican) with low dose of cyclosporine in de novo kidney recipients after 1 month of transplantation (Preliminary results). Journal of the Korean Society for Transplantation 2012;26(2):83‐91. [DOI: 10.4285/jkstn.2012.26.2.83] - DOI
-
- Oh CK, Huh KH, Ha J, Kim YH, Kim YL, Kim YS. Safety and efficacy of the early introduction of everolimus with reduced‐exposure cyclosporine a in de novo kidney recipients. Transplantation 2015;99(1):180‐6. [MEDLINE: ] - PubMed
OPTICEPT Study 2009 {published data only}
-
- Bloom R, Kaplan B, Meier‐Kriesche H, Shaw L, Shah T, Patel D, et al. Opticept Trial: Efficacy and safety of monitored MMF in combination with CNI in renal transplantation at 1 and 2 years [abstract no: 862]. Transplantation 2008;86(2 Suppl):301. [CENTRAL: CN‐00740509]
-
- Gaston R, Bloom R, Shah T, Cibrik D, Angelis M, Mulgaonkar S, et al. 6‐month outcomes of monitored mycophenolate mofetil (MMF) in combination with calcineurin inhibitors (CNI) in renal allograft recipients: an interim analysis of the OPTICEPT trial [abstract no: TH‐PO565]. Journal of the American Society of Nephrology 2006;17(Abstracts):228A. [CENTRAL: CN‐00602020]
-
- Gaston R, Kaplan B, Meier‐Kriesche H, Shaw L, Shah T, Patel D, et al. Opticept Trial: efficacy and safety of monitored MMF in combination with CNI in renal transplantation at 12 months [abstract no: 526]. American Journal of Transplantation 2008;8(Suppl 2):319. [CENTRAL: CN‐00653708]
-
- Gaston RS, Kaplan B, Shah T, Cibrik D, Shaw LM, Angelis M, et al. Fixed‐ or controlled‐dose mycophenolate mofetil with standard‐ or reduced‐dose calcineurin inhibitors: the OPTICEPT trial. American Journal of Transplantation 2009;9(7):1607‐19. [MEDLINE: ] - PubMed
-
- Kaplan B, Gaston R, Meier‐Kriesche H, Bloom R, Patel D, Shaw L. Mycophenolate mofetil may require dose modification based on weight: an analysis of MPA exposure in the OptiCept Trial [abstract no: 787]. American Journal of Transplantation 2009;9(Suppl 2):419. [CENTRAL: CN‐00794654]
ORION Study 2011 {published data only}
-
- Flechner S, Cockfield S, Grinyo J, Russ G, Wissing KM, Legendre C, et al. A randomized, open‐label study to compare the safety and efficacy of two different sirolimus (SRL) regimens with tacrolimus (TAC)+ mycophenolate mofetil (MMF) in de novo renal allograft recipients: preliminary 2‐year safety results from the Orion Trial [abstract no: 444]. Transplantation 2008;86(Suppl 2):156. [CENTRAL: CN‐00740477]
-
- Flechner S, Glyda M, Steinberg S, Harler MB. A randomized, open‐label study to compare the safety and efficacy of two different sirolimus (SRL) regimens with a tacrolimus (Tac) and mycophenolate mofetil (MMF) regimen in de novo renal allograft recipients: renal function results from the ORION study [abstract no: 1141]. American Journal of Transplantation 2007;7(Suppl 2):440. [CENTRAL: CN‐00740477]
-
- Flechner S, Glyda M, Steinberg S, Harler MB. A randomized, open‐label study to compare the safety and efficacy of two different sirolimus (SRL) regimens with a tacrolimus (Tac) and mycophenolate mofetil regimen (MMF) in de novo renal allograft recipients: acute rejection and graft survival results from the ORION study [abstract no: 52]. American Journal of Transplantation 2007;7(Suppl 2):160. [CENTRAL: CN‐00653711]
-
- Flechner S, Glyda M, Steinberg S, Harler MB, ORION Trial Investigators. A randomized, open‐label study to compare the safety and efficacy of two different sirolimus (SRL) regimens with a tacrolimus (TAC) and mycophenolate mofetil regimen (MMF) in de novo renal allograft recipients: acute rejection and graft survival results from The ORION Study [abstract no: P477]. Transplant International 2007;20(Suppl 2):209.
-
- Flechner SM, Cockfield S, Grinyo J, Russ G, Wissing KM, Legendre C, et al. A randomized, open‐label study to compare the efficacy and safety of two different sirolimus (SRL) regimens with tacrolimus (TAC) + mycophenolate mofetil (MMF) in de novo renal allograft recipients: preliminary 2‐year efficacy results from the ORION trial [abstract no: 287]. American Journal of Transplantation 2008;8(Suppl 2):254. [CENTRAL: CN‐00653710]
Pacheco‐Silva 2013 {published data only}
-
- Pacheco‐Silva A, Tonato E, Durao M Jr, Requiao‐Moura L, Arruda E, Chinen R, et al. A randomized clinical trial of early conversion from tacrolimus to everolimus in deceased donor kidney transplantation [abstract no: P460]. Transplant International 2013;26(Suppl 2):277‐8. [EMBASE: 71360073]
Paoletti 2012 {published data only}
-
- Paoletti E, Marsano L, Bellino D, Cassottana P, Cannella G. Effect of everolimus on left ventricular hypertrophy of de novo kidney transplant recipients: a 1 year, randomized, controlled trial. Transplantation 2012;93(5):503‐8. [MEDLINE: ] - PubMed
Pascual 2003 {published data only}
-
- Pascual M, Curtis J, Delmonico FL, Farrell ML, Williams WW, Kalil R, et al. A prospective, randomized clinical trial of cyclosporine reduction in stable patients greater than 12 months after renal transplantation. Transplantation 2003;75(9):1501‐5. [MEDLINE: ] - PubMed
-
- Wong W, Tolkoff‐Rubin N, Delmonico FL, Cardarelli F, Saidman SL, Farrell ML, et al. Analysis of the cardiovascular risk profile in stable kidney transplant recipients after 50% cyclosporine reduction. Clinical Transplantation 2004;18(4):341‐8. [MEDLINE: ] - PubMed
-
- Wong W, Tolkoff‐Rubin N, Delmonico FL, Farell ML, Shih V, Williams W, et al. Analysis of the cardiovascular risk profile in stable renal transplant recipients after 50% cyclosporine reduction [abstract]. American Journal of Transplantation 2003;3(Suppl 5):213. [CENTRAL: CN‐00448411]
-
- Wong W, Tolkoff‐Rubin N, Delmonico FL, Farrell ML, Shih V, Cosimi AB, et al. A randomized clinical trial to analyze the effect of 50% cyclosporine reduction on the cardiovascular risk profile in stable kidney transplant recipients [abstract]. Journal of the American Society of Nephrology 2003;14(Nov):186A. [CENTRAL: CN‐00550675]
Pascual 2008 {published data only}
Pedersen 1991 {published data only}
-
- Jensen JD, Hansen HE, Pedersen EB. Increased serum erythropoietin level during azathioprine treatment in renal transplant recipients. Nephron 1994;67(3):297‐301. [MEDLINE: ] - PubMed
-
- Pedersen EB, Hansen HE, Kornerup HJ, Madsen S, Sorensen AW. Long‐term graft survival after conversion from cyclosporin to azathioprine 1 year after renal transplantation. A prospective, randomized study from 1 to 6 years after transplantation. Nephrology Dialysis Transplantation 1993;8(3):250‐4. [MEDLINE: ] - PubMed
-
- Pedersen EB, Hansen HE, Kornerup HJ, Madsen S, Sorensen AW. Long‐term graft survival after conversion from cyclosporin to azathioprine 1 year after renal transplantation: a prospective randomized study from 1 to 6 years after transplantation [abstract]. Nephrology Dialysis Transplantation 1993;8(9):1047. - PubMed
-
- Pedersen EB, Hansen HE, Kornerup HJ, Madsen S, Sorensen AW. Long‐term graft survival after conversion from cyclosporine to azathioprine one year after renal transplantation. A prospective, randomized study from one to six years after transplantation [abstract]. 12th International Congress of Nephrology; 1993 June 13‐18; Jerusalem, Israel. 1993:194. [CENTRAL: CN‐00644352] - PubMed
-
- Pedersen EB, Hansen HE, Olsen S. The effect of cyclosporine and azathioprine on GFR and renal histopathology 12 to 24 months after renal transplantation. Transplantation Proceedings 1992;24(1):309‐10. [MEDLINE: ] - PubMed
Pontrelli 2008 {published data only}
-
- Pontrelli P, Rossini M, Infante B, Stallone G, Schena A, Loverre A, et al. Rapamycin inhibits PAI‐1 expression and reduces interstitial fibrosis and glomerulosclerosis in chronic allograft nephropathy. Transplantation 2008;85(1):125‐34. [MEDLINE: ] - PubMed
-
- Rossini M, Pontrelli P, Stallone G, Infante B, Schena A, Loverre A, et al. Regression of glomerulosclerosis in chronic allograft nephropathy (CAN) following calcineurin inhibitors (CNI) withdrawal and conversion to sirolimus (SRL) [abstract no: TH‐PO554]. Journal of the American Society of Nephrology 2006;17(Abstracts):225A. [CENTRAL: CN‐00725009]
-
- Stallone G, Pontrelli P, Infante B, Schena A, Ursi M, Ranieri E, et al. Rapamycin (RAPA) inhibits PAI‐1 expression and reduce interstitial fibrosis in chronic allograft nephropathy (CAN) [abstract no: SO04]. Nephrology Dialysis Transplantation 2005;20(Suppl 5):v4. [CENTRAL: CN‐00716118] - PubMed
Qazi 2014 {published data only}
-
- Peddi V, Qazi Y, Shaffer D, Luan F, Shihab F, Tomlanovich S, et al. Effect of everolimus with low dose tacrolimus vs mycophenolate with standard tacrolimus regimen in African‐American de novo renal transplant recipients [abstract no: B955]. Transplantation 2014;98(Suppl 1):536. [EMBASE: 71545337]
-
- Qazi Y, Shaffer D, Kaplan B, Kim D, Luan F, Peddi V, et al. Efficacy and safety of everolimus with low‐dose tacrolimus in de novo renal transplant recipients: 12‐month randomized study [abstract no: 713]. Transplantation 2014;98(Suppl 1):80. [EMBASE: 71543789] - PubMed
-
- Shihab F, Qazi Y, Kaplan B, Kim D, Mulgaonkar S, Peddi V, et al. Everolimus‐facilitated tacrolimus minimization preserves renal function in de novo renal transplant recipients [abstract no: B962]. Transplantation 2014;98(Suppl 1):538‐9. [EMBASE: 71545344]
REFERENCE Study 2006 {published data only}
-
- Frimat L, Cassuto‐Viguier E, Charpentier B, Noel C, Provot F, Rostaing L, et al. Impact of cyclosporine reduction with MMF: a randomized trial in chronic allograft dysfunction. The 'REFERENCE' study. American Journal of Transplantation 2006;6(11):2725‐34. [MEDLINE: ] - PubMed
-
- Kessler M. Renal function evaluation after half dose reduction of Neoral in combination with Cellcept in renal transplant patients with altered renal function: preliminary 12 months results: randomized, open, multicentric, prospective, controlled study [abstract no: 773]. American Journal of Transplantation 2003;3(Suppl 5):350. [CENTRAL: CN‐00446065]
-
- Kessler M, Frimat L. Renal function evaluation after half dose reduction of neoral® in combination with Cellcept® in renal transplant patients with altered renal function: preliminary 2 year safety and efficacy results of the MMF‐REFERENCE study: a randomised [abstract no: 013]. Transplantation 2004;78(2 Suppl):5. [CENTRAL: CN‐00509272]
-
- Kessler M, Frimat L, Cassuto‐Viguier E, Charpentier B, Djeffal R, Noel C, et al. Impact of cyclosporine reduction with MMF in chronic allograft dysfunction: 4‐year results of a multicenter randomized controlled study. The "Reference" study [abstract no: 850]. American Journal of Transplantation 2006;6(Suppl 2):353. [CENTRAL: CN‐00765336] - PubMed
-
- Kessler M, Frimat L, Charpentier B, Durrbach A, Noel C, Pruvot F, et al. Renal function evaluation after half dose reduction of neoral® in combination with cellcept® in renal transplant patients with altered renal function: preliminary 2 year safety and efficacy results of the MMF ‐ REFERENCE study: a randomised, open, multicentre, prospective, controlled study [abstract no: 462]. American Journal of Transplantation 2004;4(Suppl 8):285. [CENTRAL: CN‐00602100]
Rivelli 2015 {published data only}
-
- Rivelli RF, Goncalves RT, Leite M Jr, Santos MA, Delgado AG, Cardoso LR, et al. Early withdrawal of calcineurin inhibitor from a sirolimus‐based immunosuppression stabilizes fibrosis and the transforming growth factor‐beta signalling pathway in kidney transplant. Nephrology 2015;20(3):168‐76. [MEDLINE: ] - PubMed
RMR Study 2001 {published data only}
-
- Balshaw R, Marra C, Nashan B, Hagenmeyer EG, Keown P. First year cost‐implications of early cyclosporine withdrawal in renal transplantation [abstract no: 2378]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00415221]
-
- Campistol JM, Eris J, Oberbauer R, Friend P, Hutchison B, Morales JM, et al. Sirolimus therapy after early cyclosporine withdrawal reduces the risk for cancer in adult renal transplantation. Journal of the American Society of Nephrology 2006;17(2):581‐9. [MEDLINE: ] - PubMed
-
- Campistol JM, Kreis H, Oberbauer R, Mota A, Riad H, Chapman J, et al. Sirolimus‐based therapy following early cyclosporin withdrawal resulted in superior renal allograft survival at 48 months compared with continuous combined sirolimus and cyclosporine [abstract]. American Journal of Transplantation 2004;4(Suppl 8):344. [CENTRAL: CN‐00509115]
-
- Campistol JM, Legendre C, Friend P, Riad H, Castagneto M. Evaluating cardiovascular risk factors associated with long‐term sirolimus (Rapamune®) immunotherapy used with or without cyclosporine [abstract]. Nephrology Dialysis Transplantation 2002;17(Suppl 1):311. [CENTRAL: CN‐00550754]
-
- Campistol JM, Oberbauer R, Hartmann A, Kreis H, Mota A, Arias M, et al. Long‐term graft survival is significantly better in patients receiving sirolimus‐based therapy after early cyclosporine withdrawal [abstract no: W736]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):784. [CENTRAL: CN‐00444661]
Rossini 2007 {published data only}
-
- Rossini M, Loverre A, Stallone G, Infante B, Schena A, Maiorano A, et al. Proteinuria and vascular endothelial growth factor (VEGF) expression following calcineurin inhibitors (CNI) withdrawal and conversion to rapamycin [abstract no: 1149]. American Journal of Transplantation 2007;7(Suppl 2):442. [CENTRAL: CN‐00725010]
-
- Rossini M, Stallone G, Infante B, Capobianco C, Loverre A, Schena A, et al. Proteinuria and vascular endothelial growth factor (VEGF) expression following calcineurin inhibitors (CNI) withdrawal and conversion to rapamycin [abstract no: 548]. Transplant International 2007;20(Suppl 2):226.
Russ 2003 {published data only}
-
- Russ G, Campbell S, Chadban S, Eris J, O'Connell P, Pussell B, et al. Comparison of reduced‐and standard‐target concentration tacrolimus plus sirolimus in renal allograft recipients: preliminary 6‐month results [abstract]. Transplantation Society of Australia and New Zealand. 21st Annual Meeting; 2003 Apr 9‐11; Canberra, ACT. 2003:64. [CENTRAL: CN‐00447521]
-
- Russ G, Campbell S, Chadban S, Eris J, O'Connell P, Pussell B, et al. The safety and efficacy of reduced‐ and standard‐target concentration tacrolimus plus sirolimus in renal allograft recipients: preliminary 6‐month results from Australia [abstract no: SA‐PO496]. Journal of the American Society of Nephrology 2002;13(September, Program & Abstracts):364A. [CENTRAL: CN‐00447522]
-
- Russ GR, Campbell S, Chadban S, Eris J, O'Connell P, Pussell B, et al. Reduced and standard target concentration tacrolimus with sirolimus in renal allograft recipients. Transplantation Proceedings 2003;35(3 Suppl):115S‐7S. [MEDLINE: ] - PubMed
-
- Whelchel J, Paczek L, Bechstein WO, Russ G. A regimen of sirolimus and reduced‐dose tacrolimus results in improved renal allograft function: combined analysis of the North American target, European and Australian sirolimus‐tacrolimus trials [abstract]. American Journal of Transplantation 2003;3(Suppl 5):464. [CENTRAL: CN‐00448351]
Salvadori 2007 {published data only}
-
- Salvadori M, Scolari MP, Stefoni S, Bertoni E, Sandrini S, Rigotti P, et al. Randomized trial of sodium mycophenolate (MPS) and basiliximab in combination with reduced or standard cyclosporine (CsA) in old recipients of kidney transplant (KTx) [abstract no: O069]. Transplant International 2007;20(Suppl 2):20.
-
- Salvadori M, Scolari MP, Stefoni S, Bertoni E, Sandrini S, Rigotti P, et al. Randomized trial of sodium mycophenolate and basiliximab in combination with reduced or standard cyclosporine exposure in old recipients of kidney transplants from deceased donors [abstract no: 108]. Transplantation 2008;86(2 Suppl):39. [CENTRAL: CN‐00679020]
Schaefer 2006 {published data only}
-
- Schaefer HM, Kizilisik AT, Feurer I, Nylander WA, Langone AJ, Helderman JH, et al. Short‐term results under three different immunosuppressive regimens at one center. Transplantation Proceedings 2006;38(10):3466‐7. [MEDLINE: ] - PubMed
Smak Gregoor 1999 {published data only}
-
- Roodnat J, Hilbrands LB, Hene RJ, Sevaux RG, Gregoor PJ, Kal‐van Gestel JA, et al. 15 year follow‐up of a multicentre, randomised, calcineurin inhibitor (CNI) withdrawal study in kidney transplantation [abstract no: BO156]. Transplant International 2013;26(Suppl 2):83‐4. [EMBASE: 71359271]
-
- Roodnat JI, Hilbrands LB, Hene RJ, Sevaux RG, Smak Gregoor PJ, Kal‐van Gestel JA, et al. 15‐year follow‐up of a multicenter, randomized, calcineurin inhibitor withdrawal study in kidney transplantation. Transplantation 2014;98(1):47‐53. [MEDLINE: ] - PubMed
-
- Smak Gregoor PJ, Sevaux RG, Hene RJ, Hesse CJ, Hilbrands LB, Vos P, et al. Effect of cyclosporine on mycophenolic acid trough levels in kidney transplant recipients. Transplantation 1999;68(10):1603‐6. [MEDLINE: ] - PubMed
-
- Smak Gregoor PJ, Sevaux RG, Ligtenberg G, Hoitsma AJ, Hene RJ, Weimar W, et al. A prospective randomised study of withdrawal of cyclosporine or prednisone in renal transplant recipients treated with mycophenolate mofetil, cyclosporine, and prednisone: 18 months follow‐up data [abstract no: 441]. American Journal of Transplantation 2001;1(Suppl 1):246. [CENTRAL: CN‐00763745]
-
- Smak Gregoor PJ, Sevaux RG, Ligtenberg G, Hoitsma AJ, Hene RJ, Weimar W, et al. Withdrawal of cyclosporine or prednisone six months after kidney transplantation in patients on triple drug therapy: a randomized, prospective, multicenter study. Journal of the American Society of Nephrology 2002;13(5):1365‐73. [MEDLINE: ] - PubMed
SMART TX Study 2010 {published data only}
-
- Guba M, Pratschke J, Hugo C, Kraemer B, Burmeister D, Brockmann J, et al. A randomized multicenter trial of early conversion to sirolimus/mycophenolate/steroids versus cyclosporine/mycophenolate/steroids in renal transplantation: one‐year analysis (SMART‐Study) [abstract no: 1088]. American Journal of Transplantation 2009;9(Suppl 2):497. [CENTRAL: CN‐00763842]
-
- Guba M, Pratschke J, Hugo C, Kraemer B, Burmeister D, Brockmann J, et al. Comparison of a delayed low‐dose sirolimus (Siro)/mycophenolate mofetil (MMF)‐based therapy with standard cyclosporine (CsA/MMF‐based immunosuppression in patients after kidney transplantation (SMART study) [abstract no: P188]. Transplant International 2007;20(Suppl 2):141. [CENTRAL: CN‐00724890]
-
- Guba M, Pratschke J, Hugo C, Kramer B, Nohr‐Westphal C, Brockmann J. Renal function, efficacy and safety of sirolimus and mycophenolate mofetil therapy after early calcineurin‐inhibitor withdrawal in de novo renal transplant patients: one‐year analysis of a randomized multicenter trial [abstract no: O‐296]. Transplant International 2009;22(Suppl 2):78. - PubMed
-
- Guba M, Pratschke J, Hugo C, Kramer BK, Pascher A, Pressmar K, et al. Early conversion to a sirolimus‐based, calcineurin‐inhibitor‐free immunosuppression in the SMART trial: observational results at 24 and 36months after transplantation. Transplant International 2012;25(4):416‐23. [MEDLINE: ] - PubMed
-
- Guba M, Pratschke J, Hugo C, Krämer BK, Nohr‐Westphal C, Brockmann J, et al. Renal function, efficacy, and safety of sirolimus and mycophenolate mofetil after short‐term calcineurin inhibitor‐based quadruple therapy in de novo renal transplant patients: one‐year analysis of a randomized multicenter trial. Transplantation 2010;90(2):175‐83. [MEDLINE: ] - PubMed
Spare‐the‐Nephron Study 2011 {published data only}
-
- Kalil R, Pearson TC, Mulgaonkar S, Patel A, Shidban H, Weir M, et al. Final 1‐year outcomes of the spare‐the‐nephron (STN) trial: mycophenolate mofetil (MMF) ‐ based regimen combined with sirolimus (SRL) to preserve renal function in renal transplantation [abstract no: TH‐FC042]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):10A. [CENTRAL: CN‐00716075]
-
- Mulgaonkar S, Pearson TC, Patel A, Scandling J, Shidban H, Weir M, et al. Final renal function outcomes from the Spare‐the‐Nephron (STN) trial: mycophenolate mofetil (MMF)/sirolimus (SRL) maintenance therapy and CNI withdrawal in renal transplant recipients [abstract no: 530]. American Journal of Transplantation 2008;8(Suppl 2):320. [CENTRAL: CN‐00690361]
-
- Patel A, Weir MR, Wali R, Pearson T, Mulgaonkar S, Shidban H, et al. Spare‐the‐Nephron (STN) Trial: Updated analysis of renal function after one year of mycophenolate mofetil/sirolimus maintenance therapy and calcineurin inhibitor withdrawal in renal transplant recipients [abstract no: 1138]. American Journal of Transplantation 2007;7(Suppl 2):439. [CENTRAL: CN‐00615833]
-
- Pearson T, Mulgaonkar S, Kalil R, Patel A, Scandling J, Patel D, et al. CNI withdrawal in African Americans ‐ 1 year outcomes of African American renal transplant recipients in the Spare‐the‐Nephron (STN) trial [abstract no: 1083]. American Journal of Transplantation 2009;9(Suppl 2):496.
-
- Pearson TC, Mulgaonkar S, Patel A, Scandling J, Shidban H, Weir M, et al. Efficacy and safety of mycophenolate mofetil (MMF)/sirolimus (SRL) maintenance therapy after calcineurin inhibitor (CNI) withdrawal in renal transplant recipients: final results of the Spare‐the‐Nephron (STN) trial [abstract no: 129]. American Journal of Transplantation 2008;8(Suppl 2):213. [CENTRAL: CN‐00690150]
Stallone 2003 {published data only}
-
- Stallone G, Paola S, Schena A, Infante B, Grandaliano G, Battaglia M, et al. Early withdrawal of cyclosporine A improves 1‐year kidney graft structure and function in sirolimus‐treated patients. Transplantation 2003;75(7):998‐1003. [MEDLINE: ] - PubMed
-
- Stallone G, Schena A, Infante B, Grandaliano G, Gesualdo L, Schena PF, et al. Early withdrawal of cyclosporine (CSA) ameliorates 1‐year kidney graft function and structure in sirolimus (SRL)‐treated patients. [abstract no: 1014]. American Journal of Transplantation 2002;2(Suppl 3):393. [CENTRAL: CN‐00527169]
Stallone 2004 {published data only}
-
- Stallone G, Paolo S, Schena A, Infante B, Battaglia M, Ditonno P, et al. Addition of sirolimus to cyclosporine delays the recovery from delayed graft function but does not affect 1‐year graft function. Journal of the American Society of Nephrology 2004;15(1):228‐33. [MEDLINE: ] - PubMed
-
- Stallone G, Infante B, Schena A, Paolo S, Gesualdo L, Ditonno P, et al. Sirolimus prolongs delayed graft function in recipients of sub‐optimal cadaveric kidney donors [abstract]. American Journal of Transplantation 2003;3(Suppl 5):563. [CENTRAL: CN‐00447829]
Stegall 2003 {published data only}
-
- Dean PG, Grande JP, Sethi S, Park WD, Griffin MD, Cosio FG, et al. Kidney transplant histology after one year of continuous therapy with sirolimus compared with tacrolimus. Transplantation 2008;85(8):1212‐5. [MEDLINE: ] - PubMed
-
- Dean PG, Larson TS, Rea DJ, Griffin MD, Textor SC, Schwab TR, et al. The effect of immunosuppression on renal function and graft histology: a comparison of tacrolimus and sirolimus [abstract no: 379]. American Journal of Transplantation 2005;5(Suppl 11):252. [CENTRAL: CN‐00725015]
-
- Dean PG, Larson TS, Rea DJ, Griffin MD, Textor SC, Schwab TR, et al. The effects of calcineurin inhibitor avoidance on renal function and graft histology after kidney transplantation: a prospective, randomized comparison of tacrolimus and sirolimus [abstract no: 259]. Transplantation 2004;78(2 Suppl):89. [CENTRAL: CN‐00509154]
-
- Dean PG, Lund WJ, Larson TS, Prieto M, Nyberg SL, Ishitani MB, et al. Wound healing complications after kidney transplantation: a prospective, randomized comparison of sirolimus and tacrolimus [abstract]. American Journal of Transplantation 2004;4(Suppl 8):229. - PubMed
-
- Dean PG, Lund WJ, Larson TS, Prieto M, Nyberg SL, Ishitani MB, et al. Wound‐healing complications after kidney transplantation: a prospective, randomized comparison of sirolimus and tacrolimus. Transplantation 2004;77(10):1555‐61. [MEDLINE: ] - PubMed
Suwelack 2002 {published data only}
-
- Hillebrand U, Suwelack B, Gerhardt U, Kobelt V, Kraemer J, Hohage H. Impact of calcineurin inhibitor withdrawal and mycophenolate mofetil substitution on hematologic toxicity [abstract no: 0014]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00415864]
-
- Suwelack B, Diet KH, Kobelt V, Hohage H, Gerhardt U. Impact of calcineurin‐inhibitor withdrawal on arterial distensibility and endothelial function in chronic allograft nephropathy [abstract no: 1048]. American Journal of Transplantation 2002;2(Suppl 3):402. [CENTRAL: CN‐00416722]
-
- Suwelack B, Gerhardt U, Hohage H. Withdrawal of cyclosporine or tacrolimus after addition of mycophenolate mofetil in patients with chronic allograft nephropathy. American Journal of Transplantation 2004;4(4):655‐62. [MEDLINE: ] - PubMed
-
- Suwelack B, Gerhardt U, Kobelt V, Hillebrand U, Matzkies F, Hohage H. Design and preliminary results of a randomized study on the conversion of treatment with calcineurin inhibitors to mycophenolate mofetil in chronic renal graft failure: effect, on serum cholesterol levels. Transplantation Proceedings 2002;34(5):1803‐5. [MEDLINE: ] - PubMed
-
- Suwelack B, Hausberg M, Hillebrand U, Rahn KH, Hohage H. Study on effects of calcineurin inhibitor withdrawal on arterial distensibility and endothelial function in renal transplant recipients [abstract no: 0637]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00416723] - PubMed
SYMPHONY Study 2007 {published data only}
-
- Bagul A, Nicholson M, Chavez R, Grinyo J, Frei U, Vanrenterghem Y, et al. Low‐dose sirolimus in the first 8 weeks following renal transplantation accompanied by daclizumab induction, MMF and steroids: The experience of the SYMPHONY study [abstract no: P23]. British Transplantation Society (BTS).10th Annual Congress; 2007 Mar 28‐30; Manchester, UK. 2007.
-
- Chavez R, Nicholson M, Grinyo J, Frei U, Vanrenterghem Y, Daloze P, et al. SYMPHONY ‐ comparing efficacy of standard immunosuppression to low‐dose cyclosporine, tacrolimus or sirolimus in combination with MMF, daclizumab and corticosteroids in renal transplantation. Sub analysis of GFR in cases that completed one year within an intended regime [abstract no: O06]. British Transplantation Society (BTS). 10th Annual Congress; 2007 Mar 28‐30; Manchester, UK. 2007.
-
- Claes K, Meier‐Kriesche HU, Schold JD, Vanrenterghem Y, Halloran PF, Ekberg H. Effect of different immunosuppressive regimens on the evolution of distinct metabolic parameters: evidence from the Symphony study. Nephrology Dialysis Transplantation 2012;27(2):850‐7. [MEDLINE: ] - PubMed
-
- Colom H, Fernandez De Troconiz I, Caldes A, Oppenheimer F, Sanchez Plumed J, Gentil MA, et al. Population pharmacokinetics of mycophenolic acid in combination with free or reduced doses of calcineurin inhibitors during the first week in renal transplant: the Symphony Study [abstract no: 105]. Transplantation 2008;86(2 Suppl):37. [CENTRAL: CN‐00678981]
-
- Daloze P, Ekberg H, Vincenti F, Tedesco‐Silva H, Pearson T. Low‐dose sirolimus in the first 8 weeks following renal transplantation accompanied by daclizumab induction, MMF and steroids: the experience of the SYMPHONY Study [abstract no: F‐PO1078]. Journal of the American Society of Nephrology 2006;17(Abstracts):563A.
Takahashi 2013a {published data only}
-
- Saito K, Uchida K, Takahara S, Yoshimura N, Teraoka S, Cornu‐Artis C, et al. Efficacy of everolimus with reduced cyclosporine in Japanese de novo renal transplant recipients: 24‐month, randomized, multicenter study [abstract no: B944]. American Journal of Transplantation 2013;13(Suppl S5):314. [EMBASE: 71057520]
-
- Takahara S, Uchida K, Yoshimura N, Teraoka S, Kobayashi E, Teshima R, et al. Efficacy and safety of concentration controlled everolimus with reduced dose cyclosporine in Japanese adult de‐novo renal transplant patients: 12 month results [abstract no: 935]. American Journal of Transplantation 2012;12(Suppl S3):300. [EMBASE: 70746888] - PMC - PubMed
-
- Uchida K, Hoshinaga K, Watarai Y, Goto N, Kusaka M, Sasaki H, et al. Pharmacokinetics of everolimus when combined with cyclosporine in Japanese de novo renal transplant recipients. Transplantation Proceedings 2014;46(5):1314‐8. [MEDLINE: ] - PubMed
-
- Watarai Y, Akutsu N, Saito K, Nakagawa Y, Kamisawa O, Kenmochi T. Everolimus plus reduced‐exposure calcineurin inhibitor versus mycophenolate mofetil plus standard‐exposure calcineurin inhibitor: 2‐year results in living donor kidney transplant recipients [abstract]. American Journal of Transplantation 2015;15(Suppl 3). [EMBASE: 71953410]
Tedesco‐Silva 2010 {published data only}
-
- Campbell S, Walker R, Pilmore H, Kanellis J, Russ G, Hutchison B, et al. Wound healing events are dose related: a multicenter, prospective study on everolimus in renal transplantation [abstract no: 43]. Transplantation Society of Australia & New Zealand (TSANZ). 29th Annual Scientific Meeting; 2011 Jun 29‐Jul 1; Canberra (ACT). 2011:64.
-
- Carmellini M, Garcia V, Wang Z, Vergara M, Russ G. Efficacy of everolimus with reduced‐exposure cyclosporine in de novo kidney transplant patients at increased risk for efficacy events: analysis of a randomized trial. Journal of Nephrology 2015;28(5):633‐9. [MEDLINE: ] - PubMed
-
- Carmellini M, Garcia V, Wong Z, Vergara M, Escrig C, Russ G. Treatment with everolimus and reduced‐exposure cyclosporine is efficacious in de novo renal transplant recipients at increased risk for efficacy failure: Post‐Hoc analysis from the A2309 study [abstract]. American Journal of Transplantation 2015;15(Suppl 3). [EMBASE: 71954404]
-
- Chadban S, Pilmore H, Russ G, Kanellis J, Campbell S, O'Connell P, et al. Everolimus plus reduced‐exposure cyclosporin versus mycophenolic acid plus cyclosporin: seven year follow‐up of ANZ patients from a randomised controlled trial [abstract no: 079]. Nephrology 2015;30(Suppl 3):39. [EMBASE: 71995868]
-
- Cibrik D, Johnston T, Kim YS, Walker R, Zibari G, Mange K, et al. Everolimus allows for around 60% reduction in CsA exposure over 12 months: results from a multicenter, prospective study in renal transplantation [abstract no: 1667]. American Journal of Transplantation 2010;10(Suppl 4):511. [EMBASE: 70465042]
Velosa‐212 Study 2001 {published data only}
-
- Campistol JM, Alveranga D, Hricik DE, Velosa J, Grinyo JM, Mourad G, et al. Sirolimus‐treated renal transplant recipients experience improved renal function with cyclosporine elimination: one‐year results from a phase II trial [abstract]. Nephrology Dialysis Transplantation 2001;16(6):A208. [CENTRAL: CN‐00487674]
-
- Gonwa T, Alveranga D, Ancona G, Brinker K, Cambi V, Campistol J, et al. Sirolimus (Rapamune) permits early elimination of cyclosporine in recipients of cadaveric renal allografts [abstract]. Transplantation 2000;69(8 Suppl):S360. [CENTRAL: CN‐00445514]
-
- Gonwa TA. Sirolimus (SRL) treated patients demonstrate improved renal function after withdrawal of cyclosporine (CSA) [abstract]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):891A. [CENTRAL: CN‐00550647]
-
- Gonwa TA, Hricik DE, Brinker K, Grinyo JM, Schena FP, Sirolimus Renal Function Study Group. Improved renal function in sirolimus‐treated renal transplant patients after early cyclosporine elimination. Transplantation 2002;74(11):1560‐7. [MEDLINE: ] - PubMed
-
- Hricik DE, Rapamune Renal Function Study Group. Sirolimus improves renal function without increasing acute rejection after cyclosporine elimination in renal transplant patients [abstract no: 0430]. XVIII International Congress of the Transplantation Society; 2000 Aug 27‐Sep 1; Rome, Italy. 2000. [CENTRAL: CN‐00583304]
Watson 2005 {published data only}
-
- Clatworthy M, Bradley V, Wallin E, Camilleri B, Williams P, Bradley JA, et al. Sirolimus conversion post‐renal transplantation. 5 year follow up data [abstract no: P246]. British Transplantation Society (BTS).12th Annual Congress; 2009 Apr 21‐24; Liverpool, UK. 2009.
-
- Clatworthy MR, Bradley V, Bradley JA, Watson CJ. Sirolimus conversion post‐renal transplantation ‐ 5 year follow‐up data [abstract no: 1096]. American Journal of Transplantation 2009;9(Suppl 2):499. [CENTRAL: CN‐00766450]
-
- Watson CJ, Firth J, Bradley J, Williams PF, Smith JC, Palmer CR, et al. Superior renal function following conversion to sirolimus after renal transplantation ‐ preliminary results from a randomised trial [abstract]. American Journal of Transplantation 2004;4(Suppl 8):220‐1. [CENTRAL: CN‐00509555]
-
- Watson CJ, Firth J, Williams PF, Bradley JR, Pritchard N, Chaudhry A, et al. A randomized controlled trial of late conversion from CNI‐based to sirolimus‐based immunosuppression following renal transplantation. American Journal of Transplantation 2005;5(10):2496‐503. [MEDLINE: ] - PubMed
-
- Willcocks LC, Chaudhry AN, Smith JC, Ojha S, Doffinger R, Watson CJ, et al. The effect of sirolimus therapy on vaccine responses in transplant recipients. American Journal of Transplantation 2007;7(8):2006‐11. [MEDLINE: ] - PubMed
ZEUS Study 2011 {published data only}
-
- Arns W, Becker T, Budde K, Eisenberger U, Fischer W, Kramer S, et al. Conversion to an everolimus/enteric‐coated mycophenolate sodium regimen in de novo renal transplant improves 2 year renal function [abstract no: 15]. British Transplantation Society (BTS). 14th Annual Congress; 2011 Mar 9‐11; Bournemouth, UK. 2011.
-
- Arns W, Budde K, Becker T, Sommerer C, Reinke P, Eisenberger U, et al. Analysis of renal function in everolimus/enteric‐coated mycophenolate sodium treated de novo renal transplant recipients after calcineurin inhibitor withdrawal: The ZEUS Study [abstract no: SU656]. World Congress of Nephrology; 2009 May 22‐26; Milan, Italy. 2009. [CENTRAL: CN‐00763609]
-
- Becker T, Arns W, Budde K, Eisenberger U, Fischer W, Kramer S, et al. Renal function in everolimus/enteric‐coated mycophenolate sodium treated de novo renal transplant recipients after calcineurin inhibitor withdrawal: the Zeus Study [abstract no: O‐297]. Transplant International 2009;22(Suppl 2):78‐9.
-
- Becker T, Arns W, Budde K, Pietruck F, Eisenberger U, Fischer W, et al. Improved renal function of an everolimus/enteric‐coated mycophenolate sodium regimen after calcineurin inhibitor withdrawal in de novo renal transplant patients: 2 years follow‐up of the ZEUS trial [abstract no: 1757]. Transplantation 2010;90(Suppl 2S):109. [EMBASE: 71531312]
-
- Budde K, Arns W, Sommerer C, Reinke P, Eisenberger U, Fischer W, et al. Improved renal function of an everolimus/enteric‐coated mycophenolate sodium regimen after calcineurin inhibitor withdrawal in de novo renal transplant patients: 2 years follow‐up of the ZEUS trial [abstract no: 1638]. American Journal of Transplantation 2010;10(Suppl 4):503.
References to studies excluded from this review
Abouna 1991 {published data only}
-
- Abouna GM, Kumar SM, White AG, Samhan M, Kalawi M, al‐Sabawi N. Cyclosporine withdrawal in renal transplant recipients maintained on triple therapy. Transplantation Proceedings 1991 Feb;23(1 (Pt 2)):1009‐10. [MEDLINE: ] - PubMed
Alexander 2006 {published data only}
-
- Alexander JW, Goodman HR, Cardi M, Austin J, Goel S, Safdar S, et al. Simultaneous corticosteroid avoidance and calcineurin inhibitor minimization in renal transplantation. Transplant International 2006;19(4):295‐302. [MEDLINE: ] - PubMed
Alpay 2013 {published data only}
-
- Alpay N. Conversion from calcineurin inhibitors to everolimus resulted in decrease of serum TGF‐beta and urinary NGAL in renal transplant recipients [abstract]. Nephrology Dialysis Transplantation 2013;28(Suppl 1):i500‐1. [EMBASE: 71076549]
-
- Alpay N, Ozkok A, Caliskan Y, Akagun T, Cinar S, Deniz G, et al. Conversion from calcineurin inhibitors to everolimus resulted in decrease of serum TGF‐beta and urinary NGAL in renal transplant recipients [abstract no: 1744]. American Journal of Transplantation 2013;13(Suppl 5):545. [EMBASE: 71058320]
Artz 2002 {published data only}
-
- Artz MA, Boots JM, Ligtenberg G, Roodnat JI, Christiaans MH, Hene RJ, et al. Randomized conversion from cyclosporine to tacrolimus in renal transplant patients: improved lipid profile and unchanged plasma homocysteine levels. Transplantation Proceedings 2002;34(5):1793‐4. [MEDLINE: ] - PubMed
-
- Artz MA, Boots JM, Ligtenberg G, Roodnat JI, Christiaans MH, Vos PF, et al. Conversion from cyclosporine to tacrolimus improves quality‐of‐life indices, renal graft function and cardiovascular risk profile. American Journal of Transplantation 2004;4(6):937‐45. [MEDLINE: ] - PubMed
-
- Artz MA, Boots JM, Ligtenberg G, Roodnat JI, Christiaans MH, Vos PF, et al. Improved cardiovascular risk profile and renal function in renal transplant patients after randomized conversion from cyclosporine to tacrolimus. Journal of the American Society of Nephrology 2003;14(7):1880‐8. [MEDLINE: ] - PubMed
-
- Artz MA, Boots JM, Ligtenberg G, Roodnat JI, Christiaans MH, Vos PF, et al. Randomized conversion from cyclosporine to tacrolimus improves renal graft function, the cardiovascular risk profile, and perceived side‐effects [abstract]. 3rd International Congress on Immunosuppression; 2004 Dec 8‐11; San Diego (CA). 2004. [CENTRAL: CN‐00550511]
-
- Artz MA, Ligtenberg G, Roodnat JI, Christiaans MH, Boots HM, Hene RJ, et al. Randomized conversion from cyclosporine to tacrolimus in renal transplant patients: improved parameters of lipid metabolism and unchanged plasma homocysteine levels [abstract]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):876A. [CENTRAL: CN‐00433618]
Asberg 2013 {published data only}
-
- Asberg A, Apeland T, Reisaeter AV, Foss A, Leivestad T, Heldal K, et al. Long‐term outcomes after cyclosporine or mycophenolate withdrawal in kidney transplantation ‐ results from an aborted trial. Clinical Transplantation 2013;27(2):E151‐6. [MEDLINE: ] - PubMed
Baboolal 2003 {published data only}
-
- Baboolal K. A phase III prospective, randomized study to evaluate concentration‐controlled sirolimus (Rapamune) with cyclosporine dose minimization or elimination at six months in de novo renal allograft recipients. Transplantation 2003;75(8):1404‐8. [MEDLINE: ] - PubMed
Baboolal 2004 {published data only}
-
- Baboolal K. Six month interim analysis of a phase III prospective, randomized study to compare conversion from calcineurin inhibitors to rapamycin in established renal allograft recipients with mild to moderate renal insufficiency [abstract]. American Journal of Transplantation 2004;4(Suppl 8):220. [CENTRAL: CN‐00509073]
-
- Baboolal K, Zaiac M, Newstead C. Development of early malignant disease in a multicentre, randomised study comparing conversion from calcineurin inhibitors (CNIs) to sirolimus in renal allograft recipients [abstract no: P302]. British Transplantation Society (BTS). 12th Annual Congress; 2009 Apr 21‐24; Liverpool, UK. 2009.
-
- Baboolal K, Zaiac M, Zamauskaite A, Newstead C. This multicentre, randomised study comparing conversion from calcineurin inhibitors (CNRs) to sirolimus versus standard therapy in renal allograft recipients showed a lower rate of development of subsequent malignant disease in the group receiving sirolimus [abstract no: 164]. American Journal of Transplantation 2009;9(Suppl 2):238. [CENTRAL: CN‐00776739]
Baxter 1982 {published data only}
-
- Baxter CR, Duggin GG, Willis N, Hall BM, et al. Cyclosporin A in renal transplantation: plasma concentration and nephrotoxicity [abstract]. Australian & New Zealand Journal of Medicine 1982;12:347‐8. [CENTRAL: CN‐00319765]
Brady 1990 {published data only}
-
- Brady HR, Kamel KS, Harding ME, Cook GT, deVeber GA, Cardella CJ. Low dose ciclosporin from the early postoperative period yields potent immunosuppression after renal transplantation. Nephron 1990;55(4):394‐9. [MEDLINE: ] - PubMed
Burkhalter 2012 {published data only}
-
- Burkhalter F, Oettl T, Descoeudres B, Bachmann A, Guerke L, Mihatsch MJ, et al. High incidence of rejection episodes and poor tolerance of sirolimus in a protocol with early steroid withdrawal and calcineurin inhibitor‐free maintenance therapy in renal transplantation: experiences of a randomized prospective single‐center study. Transplantation Proceedings 2012;44(10):2961‐5. [MEDLINE: ] - PubMed
-
- Oettl T, Descoeudres B, Burkhalter F, Bachmann A, Gürke L, Mihatsch MJ, et al. An open, single centre, prospective study to investigate a steroid free immunosuppressive regimen for de novo renal transplant recipients followed by a two arm randomisation to a CNI‐sparing and a CNI‐free maintenance immunosuppression after 3 months [abstract no: 38]. Swiss Medical Weekly 2008;138(Suppl 167):15S.
CAMPASIA Study 2005 {published data only}
-
- Munoz AS, Cabanayan‐Casasola CB, Danguilan RA, Padua FB, Ona ET. Campath‐1H (alemtuzumab) as an induction agent for the prevention of graft rejection and preservation of renal function in kidney transplant patients: Philippine 3‐year follow‐up. Transplantation Proceedings 2008;40(7):2230‐3. [MEDLINE: ] - PubMed
-
- Vathsala A, CAMPASIA Study Group. One year results of a pilot randomised controlled trial of the effectiveness of alemtuzumab as an induction agent for prevention of graft rejection and preservation of renal function in patients receiving kidney transplants [abstract no: T‐PO50029]. Nephrology 2005;10(Suppl 1):A215. [CENTRAL: CN‐00583367]
-
- Vathsala A, CAMPASIA Study Group. Safety and efficacy of campath‐1h (mabcampath®) with low dose cyclosprorine monotherapy in patients receiving kidney transplants ‐ 6 month analysis of the pilot randomised controlled [abstract no: O145]. Transplantation 2004;78(2 Suppl):56. [CENTRAL: CN‐00509538]
-
- Vathsala A, Ona ET, Tan S, Suresh S, Chan Y, Lou H, et al. CAMPASIA: a pilot randomised controlled trial of the effectiveness of campath‐1h (mabcampath®) as an induction agent for prevention of graft rejection and preservation of renal function in patients receiving kidney transplants. [abstract no: 908]. American Journal of Transplantation 2004;4(Suppl 8):406. [CENTRAL: CN‐00509539]
-
- Vathsala A, Ona ET, Tan SY, Suresh S, Lou HX, Cabanayan Casasola CB, et al. Lymphocyte recovery after depletion by alemtuzumab in renal transplant recipients: impact on outcome [abstract no: 1015]. American Journal of Transplantation 2005;5(Suppl 11):415. [CENTRAL: CN‐00644284]
Cattaneo 2005 {published data only}
-
- Cattaneo D, Merlini S, Zenoni S, Baldelli S, Gotti E, Remuzzi G, et al. Influence of co‐medication with sirolimus or cyclosporine on mycophenolic acid pharmacokinetics in kidney transplantation. American Journal of Transplantation 2005;5(12):2937‐44. [MEDLINE: ] - PubMed
-
- Cattaneo D, Merlini S, Zenoni S, Baldelli S, Perico N, Gotti E, et al. Effects of sirolimus on the pharmacokinetics of mycophenolic acid in kidney transplantation [abstract no: 650]. American Journal of Transplantation 2005;5(Suppl 11):322. [CENTRAL: CN‐00724878] - PubMed
Chapman 1985 {published data only}
-
- Chapman JR, Griffiths D, Harding NG, Morris PJ. Reversibility of cyclosporin nephrotoxicity after three months' treatment. Lancet 1985;325(8421):128‐30. [MEDLINE: ] - PubMed
-
- Chapman JR, Marcen R, Arias M, Raine AE, Dunnill MS, Morris PJ. Hypertension after renal transplantation. A comparison of cyclosporine and conventional immunosuppression. Transplantation 1987;43(6):860‐4. [MEDLINE: ] - PubMed
-
- Higgins RM, Richardson AJ, Endre ZH, Frostick SP, Morris PJ. Hypophosphataemia after renal transplantation: relationship to immunosuppressive drug therapy and effects on muscle detected by 31P nuclear magnetic resonance spectroscopy. Nephrology Dialysis Transplantation 1990;5(1):62‐8. [MEDLINE: ] - PubMed
-
- Morris PJ, Chapman JR, Allen RD, Ting A, Thompson JF, Dunnill MS, et al. Cyclosporin conversion versus conventional immunosuppression: long‐term follow‐up and histological evaluation. Lancet 1987;1(8533):586‐91. [MEDLINE: ] - PubMed
CIS Trial 2014 {published data only}
-
- Sommerer C, Schaier M, Morath C, Schwenger V, Rauch G, Giese T, et al. The Calcineurin Inhibitor‐Sparing (CIS) Trial ‐ individualised calcineurin‐inhibitor treatment by immunomonitoring in renal allograft recipients: protocol for a randomised controlled trial. Trials [Electronic Resource] 2014;15:489. [MEDLINE: ] - PMC - PubMed
CONCERTO Study 2005 {published data only}
-
- Curtis JJ, Thervet E, Vincenti F, Rodriguez A, Soergel M, Barbeito R. Outcomes of 117 cases of delayed graft function from two clinical trials involving early C2 monitored neoral and antibody therapy [abstract]. Transplantation 2004;78(2 Suppl):5. [CENTRAL: CN‐00509145]
-
- Mendez R, Light J, Pearson TC, Wu YM, Curtis J, Vincenti F. Neoral® therapy optimized by C2 monitoring and Simulect® induction can result in low acute rejection rate in renal transplant recipients [abstract]. American Journal of Transplantation 2004;4(Suppl 8):255. [CENTRAL: CN‐00509350]
-
- Vincenti F, Mendez R, Curtis J, Light J, Pearson T, Wu YM, et al. A multicenter, prospective study of C2‐monitored cyclosporine microemulsion in a U.S. population of de novo renal transplant recipients. Transplantation 2005;80(7):910‐6. [MEDLINE: ] - PubMed
David‐Neto 2001 {published data only}
-
- David‐Neto E, Cristiane A, Ianhez L, Nahas W, Zita B. A randomized, open‐label, prospective study comparing two different CYA‐AUC for the prevention of rejection in renal transplanted patients with and without induction therapy [abstract no: 1398]. A Transplant Odyssey; 2001 Aug 20‐23; Istanbul, Turkey. 2001. [CENTRAL: CN‐00444997]
de Sandes Freitas 2011 {published data only}
-
- Sandes Freitas TV, Harada KM, Felipe CR, Galante NZ, Sampaio EL, Ikehara E, et al. Steroid or tacrolimus withdrawal in renal transplant recipients using sirolimus. International Urology & Nephrology 2011;43(4):1221‐8. [MEDLINE: ] - PubMed
de Sevaux 1998 {published data only}
-
- Sevaux RG, Hilbrands LB, Tiggeler RG, Koene RA, Hoitsma AJ. A randomised, prospective study on the conversion from cyclosporine‐prednisone to cyclosporine‐azathioprine at 6 months after renal transplantation. Transplant International 1998;11 Suppl 1:S322‐4. [MEDLINE: ] - PubMed
-
- Sévaux RG, Hoitsma AJ, Hilbrands LB, Tiggeler RG, Koene RA. A randomised study on the effects of conversion of cyslosporine/prednisone to cyclosporine/azathioprine 6 months after kidney transplantation [abstract no: 234]. Transplantation 1998;65(12):S61. [CENTRAL: CN‐00583516] - PubMed
EVEREST Study 2009 {published data only}
-
- Citterio F, Scolari MP, Salvadori M, Castagneto M, Rigotti P, Albertazzi A, et al. A randomized trial comparing standard everolimus plus cyclosporine with higher blood everolimus levels plus very low cyclosporine levels in renal transplant recipients: preliminary results of the Everest Study [abstract no: P118]. Transplant International 2007;20(Suppl 2):124. [CENTRAL: CN‐00724884]
-
- Corbetta G, Salvadori M, Scolari MP, Citterio F, Rigotti P, Cossu M, et al. Exposure to everolimus, and not to cyclosporine, is associated with freedom from acute rejection in de novo renal recipients [abstract no: 447]. Transplantation 2008;86(Suppl 2):157.
-
- Ponticelli C, Salvadori M, Scolari MP, Citterio F, Rigotti P, Veneziano A, et al. Everolimus and minimization of cyclosporine in renal transplantation: 24‐month follow‐up of the EVEREST study. Transplantation 2011;91(10):e72‐3. [MEDLINE: ] - PubMed
-
- Salvadori M, Scolari M, Bertoni E, Citterio F, Rigotti P, Cossu M, et al. Targeting upper everolimus blood levels with very low‐dose cyclosporine is effective and safe in de novo renal transplantation [abstract no: 448]. Transplantation 2008;86(Suppl 2):158. [CENTRAL: CN‐00740453]
-
- Salvadori M, Scolari MP, Bertoni E, Citterio F, Rigotti P, Cossu M. Upper everolimus blood levels with very low‐dose cyclosporin: 12 months follow up of the Everest Study [abstract no: 1085]. American Journal of Transplantation 2009;9(Suppl 2):497. [CENTRAL: CN‐00776867]
Flechner 2004 {published data only}
-
- Flechner SM, Goel M, Feng J, Mastroianni B, Savaas K, Arnovitz J, et al. The effect of 2‐gram vs 1‐gram concentration controlled mycophenolate mofetil on transplant outcomes using sirolimus based calcineurin inhibitor drug‐free immunosuppression [abstract no: 214]. 3rd International Congress on Immunosuppression; 2004 Dec 8‐11; San Diego (CA). 2004. [CENTRAL: CN‐00550626]
Fleming 2016 {published data only}
-
- Fleming J, Taber D, Pilch N, Meadows H, Mardis C, McGillicuddy J, et al. mTOR‐based CNI minimization vs withdrawal in African American kidney transplant recipients [abstract]. American Journal of Transplantation 2015;15(Suppl 3). [EMBASE: 71953883]
-
- Fleming JN, Taber DJ, Pilch NA, McGillicuddy JW, Srinivas TR, Baliga PK, et al. A randomized, prospective comparison of transition to sirolimus‐based CNI‐minimization or withdrawal in African American kidney transplant recipients. Clinical Transplantation 2016;30(5):528‐33. [MEDLINE: ] - PubMed
Forwell 1986 {published data only}
-
- Forwell MA, Bradley JA. Low‐dose cyclosporin or azathioprine one year after renal transplantation [abstract]. Nephrology Dialysis Transplantation 1986;1(2):138. [CENTRAL: CN‐00260295] - PubMed
Fries 1988 {published data only}
-
- Fries D, Hiesse C, Charpentier B, Benoit G. Triple combination of low‐dose cyclosporin, azathioprine and steroids in first‐cadaver allografts [abstract]. Nephrology Dialysis Transplantation 1988;3(1):96. [CENTRAL: CN‐00260361] - PubMed
-
- Fries D, Hiesse C, Charpentier B, Lantz O, Bensadoun H, Benoit G. A single center experience with "low‐dose" cyclosporine in cadaveric renal transplantation. Clinical Transplants 1988:115‐29. [MEDLINE: ] - PubMed
-
- Fries D, Hiesse C, Santelli G, Gardin JP, Cantarovich M, Lantz O, et al. Triple therapy with low‐dose cyclosporine, azathioprine, and steroids: long‐term results of a randomized study in cadaver donor renal transplantation. Transplantation Proceedings 1988;20(3 Suppl 3):130‐5. [MEDLINE: ] - PubMed
Fries 1988a {published data only}
-
- Fries D. Optimal results in cadaver renal transplantation using prophylactic ALG, cyclosporin (CsA) and prednisone (P) [abstract]. Nephrology Dialysis Transplantation 1988;3(1):95. [CENTRAL: CN‐00260355] - PubMed
Fruchaud 1996 {published data only}
-
- Fruchaud G, Buisson C, Abbou C, Desvaux D, Baron C, Benmaadi A, et al. Prospective randomized study of quadruple versus triple therapy in long‐term kidney allografts. Transplantation Proceedings 1996;28(5):2819. [MEDLINE: ] - PubMed
Gaber 2003 {published data only}
-
- Gaber AO, Egidi MF, Lo A, Gaber LW, Shokouh‐Amiri MH, Grewal HP, et al. Defining the optimal doses of sirolimus and tacrolimus in high‐risk cadaveric renal transplant recipients [abstract no: 541]. American Journal of Transplantation 2002;2(Suppl 3):274. [CENTRAL: CN‐00415690]
-
- Lo A, Egidi MF, Gaber LW, Amiri HS, Vera S, Nezakatgoo N, et al. Comparison of sirolimus‐based calcineurin inhibitor‐sparing and calcineurin inhibitor‐free regimens in cadaveric renal transplantation. Transplantation 2004;77(8):1228‐35. [MEDLINE: ] - PubMed
-
- Lo A, Egidi MF, Gaber LW, Gaber AO. Observations on the use of sirolimus and tacrolimus in high‐risk renal transplant recipients. Transplantation Proceedings 2003;35(3 Suppl):105S‐8S. [MEDLINE: ] - PubMed
-
- Lo A, Egidi MF, Gaber LW, Shokouh‐Amiri MH, Nazakatgoo N, Fisher JS, et al. Observations regarding the use of sirolimus and tacrolimus in high‐risk cadaveric renal transplantation. Clinical Transplantation 2004;18(1):53‐61. [MEDLINE: ] - PubMed
Gelens 2006 {published data only}
-
- Gelens M, Christiaans M, Hooff JV. Calcineurin‐free immunosuppression and limited steroid exposure in renal transplantation [abstract no: P‐3]. 3rd International Congress on Immunosuppression; 2004 Dec 8‐11; San Diego (CA). 2004. [CENTRAL: CN‐00583729]
-
- Gelens M, Christiaans M, Hooff JV. Incidence of post transplant diabetes mellitus during calcineurin‐free and calcineurin‐based immunosuppression with limited steroid exposure [abstract no: 1210]. American Journal of Transplantation 2005;5(Suppl 11):465.
-
- Gelens M, Christianns M, Heurn EV, Hooff JV. Calcineurin‐free immunosuppression and limited steroid exposure in renal transplantation [abstract no: 865]. American Journal of Transplantation 2005;5(Suppl 11):376.
-
- Gelens MA, Christiaans MH, Heurn EL, Berg‐Loonen EP, Peutz‐Kootstra CJ, Hooff JP. High rejection rate during calcineurin inhibitor‐free and early steroid withdrawal immunosuppression in renal transplantation. Transplantation 2006;82(9):1221‐3. [MEDLINE: ] - PubMed
Ghafari 2007 {published data only}
-
- Ghafari A, Makhdoomi K, Ahmadpoor P. Early low dose versus high dose cyclosporine A (CSA) induction protocols in renal transplantation [abstract no: SP742]. Nephrology Dialysis Transplantation 2006;21(Suppl 4):iv265. - PubMed
-
- Ghafari A, Makhdoomi K, Ahmadpoor P, Afshari AT, Farshid B, Fallah MM, et al. Mycophenolate mofetil (MMF) plus low dose versus high dose cyclosporine A (CSA) induction protocols in renal transplantation [abstract no: T‐PO50034]. Nephrology 2005;10(Suppl):A216.
-
- Ghafari A, Makhdoomi K, Ahmadpour P, Afshari AT, Fallah MM, Rad PS. Low‐dose versus high‐dose cyclosporine induction protocols in renal transplantation. Transplantation Proceedings 2007;39(4):1219‐22. [MEDLINE: ] - PubMed
Gotti 2003 {published data only}
-
- Gotti E, Perico N, Perna A, Gaspari F, Cattaneo D, Caruso R, et al. Renal transplantation: can we reduce calcineurin inhibitor/stop steroids? Evidence based on protocol biopsy findings. Journal of the American Society of Nephrology 2003;14(3):755‐66. [MEDLINE: ] - PubMed
Griffin 1993 {published data only}
-
- Griffin PJ, Moore RH, Krishnan H, Fenn N, Salaman JR. Is there an optimal time for the first cyclosporin dose in renal transplantation?. Transplant International 1993;6(4):223‐5. [MEDLINE: ] - PubMed
Grino 1991 {published data only}
-
- Gonzalez C, Grino JM, Castelao AM, Seron D, Gil‐Vernet S, Andres E, et al. Pre‐transplant ALG, low dose cyclosporine (CsA) and steroids versus pre‐transplant OKT3, CsA and steroids in kidney cadaveric transplantation [abstract]. Kidney International 1990;37(6):1601. [CENTRAL: CN‐00601919]
-
- Grino JM, Castelao AM, Gonzalez C, Seron D, Gil‐Vernet S, Andres E, et al. Pre‐transplant ALG, low dose cyclosporine (CYA) and steroids in kidney cadaveric transplantation [abstract]. 11th International Congress of Nephrology; 1990 Jul 15‐20; Tokyo, Japan. 1990:513A. [CENTRAL: CN‐00601920]
-
- Grino JM, Castelao AM, Seron D, Gonzalez C, Galceran JM, Gil‐Vernet S, et al. Antilymphocyte globulin versus OKT3 induction therapy in cadaveric kidney transplantation: a prospective randomized study. American Journal of Kidney Diseases 1992;20(6):603‐10. [MEDLINE: ] - PubMed
-
- Grino JM, Castelao AM, Seron D, Gonzalez C, Galceran JM, Gil‐Vernet S, et al. Prophylactic OKT3, CyA, and steroids versus antilymphoblast globulin, CyA, and steroids in cadaveric kidney transplantation. Transplantation Proceedings 1992;24(1):39‐41. [MEDLINE: ] - PubMed
-
- Grino JM, Castelao AM, Seron D, Gonzalez C, Gil‐Vernet S, Andres E, et al. Antilymphocyte serum, cyclosporine and corticoids, versus OKT3, cyclosporine, and corticoids in kidney transplantation [Serum anti‐lymphocyte, ciclosporine et corticoides, versus OKT3, ciclosporine et corticoides en transplantation renale]. Presse Medicale 1991;20(40):2039‐42. [MEDLINE: ] - PubMed
Hamdy 2005 {published data only}
-
- Hamdy A, El‐Baz M, Bakr M, Ghoneim M. The incidence of chronic allograft nephropathy among live‐donor renal transplant recipients primarily treated with sirolimus‐based regimens [abstract no: SA734]. World Congress of Nephrology; 2009 May 22‐26; Milan, Italy. 2009. [CENTRAL: CN‐00776624]
-
- Hamdy AF, Bakr MA, Ghoneim MA. Proteinuria among primarily sirolimus treated live‐donor renal transplant recipients' long‐term experience. Experimental & Clinical Transplantation: Official Journal of the Middle East Society for Organ Transplantation 2010;8(4):283‐91. [MEDLINE: ] - PubMed
-
- Hamdy AF, El‐Agroudy AE, Bakr MA, Mostafa A, El‐Baz M, El‐Shahawy el‐M, et al. Comparison of sirolimus with low‐dose tacrolimus versus sirolimus‐based calcineurin inhibitor‐free regimen in live donor renal transplantation. American Journal of Transplantation 2005;5(10):2531‐8. [MEDLINE: ] - PubMed
Hariran 2015 {published data only}
-
- Haririan A, Klassen D, Keshtkar M, Drachenberg C, Dowling T, Ramos E, et al. Delayed tacrolimus (TAC) to rapamycin (RAPA) conversion in renal transplant recipients with DGF/SGF [abstract]. American Journal of Transplantation 2015;15(Suppl 3). [EMBASE: 71953312]
Henny 1986 {published data only}
-
- Henny FC, Kootte AM, Bockel JH, Baldwin WM, Hermans J, Bos B, et al. A prospective randomised comparative study on the influence of cyclosporin and azathioprine on renal allograft survival and function. Nephrology Dialysis Transplantation 1986;1(1):44‐9. [MEDLINE: ] - PubMed
-
- Hollander AA, Saase JL, Kootte AM, Dorp WT, Bockel HJ, Es LA, et al. Beneficial effects of conversion from cyclosporin to azathioprine after kidney transplantation. Lancet 1995;345(8950):610‐4. [MEDLINE: ] - PubMed
-
- Hollander AM, Saase JL, Kootte AM, Dorp WT, Bockel HH, Es LA, et al. Conversion from cyclosporine (CYA) to azathioprine (AZA) after cadaveric kidney transplantation (KT): in the long run a better renal function, less hypertension and equal graft survival [abstract no: 8P]. Journal of the American Society of Nephrology 1993;4(Program & Abstracts):941. [CENTRAL: CN‐00484382]
-
- Kootte AM, Lensen LM, Es LA, Paul LC. Controlled cyclosporine conversion at three months after renal transplantation. Long‐term results. Transplantation 1988;46(5):677‐80. [MEDLINE: ] - PubMed
-
- Kootte AM, Lensen LM, Bockel JH, Paul LC. A randomized study comparing high‐ and low‐dose regimens of cyclosporine in renal transplantation. Transplantation Proceedings 1988;20(3 Suppl 3):136‐9. [MEDLINE: ] - PubMed
Hernandez 2007 {published data only}
-
- Hernandez D, Miquel R, Porrini E, Fernandez A, Gonzalez‐Posada JM, Hortal L, et al. Randomized controlled study comparing reduced calcineurin inhibitors exposure versus standard cyclosporine‐based immunosuppression. Transplantation 2007;84(6):706‐14. [MEDLINE: ] - PubMed
Hiesse 1991 {published data only}
-
- Hiesse C, Neyrat N, Deglise‐Favre A, Lantz O, Bensadoun H, Benoit G, et al. Randomized prospective trial of elective cyclosporine withdrawal from triple therapy at 6 months after cadaveric renal transplantation. Transplantation Proceedings 1991;23(1 (Pt 2)):987‐9. [MEDLINE: ] - PubMed
Hilbrands 1993 {published data only}
-
- Hilbrands LB, Demacker PN, Hoitsma AJ. Cyclosporin and serum lipids in renal transplant recipients. Lancet 1993;341(8847):765‐6. [MEDLINE: ] - PubMed
-
- Hilbrands LB, Demacker PN, Hoitsma AJ, Stalenhoef AF, Koene RA. The effects of cyclosporine and prednisone on serum lipid and (apo)lipoprotein levels in renal transplant recipients. Journal of the American Society of Nephrology 1995;5(12):2073‐81. [MEDLINE: ] - PubMed
-
- Hilbrands LB, Hoitsma AJ, Koene KA. Randomized, prospective trial of cyclosporine monotherapy versus azathioprine‐prednisone from three months after renal transplantation. Transplantation 1996;61(7):1038‐46. [MEDLINE: ] - PubMed
-
- Hilbrands LB, Hoitsma AJ, Koene RA. Costs of drugs used after renal transplantation. Transplant International 1996;9 Suppl 1:S399‐402. [MEDLINE: ] - PubMed
-
- Hilbrands LB, Hoitsma AJ, Koene RA. Effect of immunosuppressive therapy on quality of life after renal transplantation [abstract]. Journal of the American Society of Nephrology 1994;5(3):1011. [CENTRAL: CN‐00615875]
Hourmant 1987 {published data only}
-
- Hourmant M, Buzelin F, Dubigeon P, Soulillou JP. High long‐term graft survival rates in kidney transplantation with the sequential association of antithymocyte globulin and cyclosporine A monotherapy. Transplantation Proceedings 1987;19(1 (Pt 3)):2113‐4. [MEDLINE: ] - PubMed
-
- Hourmant M, Soulillou JP. Use of delayed cyclosporin A in after administration of anti‐lymphocyte serum in kidney transplantation [Utilisation de la cyclosporine A en relais du serum anti‐lymphocytaire en transplantation renale]. Biomedicine & Pharmacotherapy 1987;41(5):247‐9. [MEDLINE: ] - PubMed
Hricik 1990 {published data only}
-
- Hricik DE, Mayes JT, Schulak JA. Cyclosporine (CsA) inhibits the generation of antibodies (ABs) to OKT3 [abstract]. Kidney International 1990;37:607. [CENTRAL: CN‐00626073]
-
- Hricik DE, Mayes JT, Schulak JA. Inhibition of anti‐OKT3 antibody generation by cyclosporine‐‐results of a prospective randomized trial. Transplantation 1990;50(2):237‐40. [MEDLINE: ] - PubMed
Infante 2008 {published data only}
-
- Infante B, Stallone G, Pontrelli P, Gigante M, Ranieri E, Schena FP, et al. Role of rapamycin in the induction of operational tolerance through up‐regulation of ILT3 and ILT4 in kidney transplanted patients [abstract no: F‐FC248]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):56A. [CENTRAL: CN‐00716071]
Jain 2001 {published data only}
-
- Jain S, Metcalfe M, White SA, Furness PN, Nicholson ML. Chronic allograft nephropathy: a prospective randomised trial of cyclosporin reduction with or without mycophenolate mofetil. Transplantation Proceedings 2001;33(3):2165‐6. [MEDLINE: ] - PubMed
-
- Jain S, Metcalfe M, White SA, Furness PN, Nicholson ML. Randomized trial comparing mycophenolate mofetil and azathioprine to allow cyclosporin reduction in chronic allograft nephropathy [abstract no: PO513W]. XVIII International Congress of the Transplantation Society; 2000 Aug 27‐Sep 1; Rome, Italy. 2000. [CENTRAL: CN‐00445890]
-
- Nicholson ML, Jain S, Metcalfe M, White SA, Furness PN. Chronic allograft nephropathy: a prospective randomised trial of cyclosporin reduction with or without mycophenolate mofetil [abstract no: 437]. Transplantation 2000;69(8 Suppl):S227. [CENTRAL: CN‐00446949] - PubMed
Jindal 2002 {published data only}
-
- Jardine A. 12 month results of a phase III prospective, randomised study to evaluate concentration controlled Rapamune with cyclosporin dose minimization or elimination in de novo renal allograft recipient [abstract no: 1206]. American Journal of Transplantation 2003;3(Suppl 5):461. [CENTRAL: CN‐00445901] - PubMed
-
- Jardine A, European and South African Rapamune Study Group. Phase III prospective, randomized study to evaluate the safety and efficacy of concentration controlled Rapamune (sirolimus) with cyclosporine dose minimisation or elimination in de novo renal allograft recipients at 12 months [abstract no: O81]. Transplantation 2004;78(2 Suppl):31. [CENTRAL: CN‐00509251]
-
- Jardine AG. Phase III prospective, randomized study to evaluate the safety and efficacy of concentration controlled Rapamune (sirolimus) with cyclosporin dose minimisation or elimination in de novo renal allograft recipients at 12 months [abstract]. American Journal of Transplantation 2004;4(Suppl 8):286. [CENTRAL: CN‐00520346] - PubMed
-
- Jindal RM, UK and Ireland Rapamune Study Group. A phase III prospective, randomised study to evaluate concentration controlled rapamune with cyclosporin dose minimization or elimination at six months in de novo renal allograft recipients [abstract]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00415940] - PubMed
John 1999 {published data only}
-
- John GT, Dakshinamurthy DS, Jeyaseelan L, Jacob CK. The effect of cyclosporin A on plasma lipids during the first year after renal transplantation. National Medical Journal of India 1999;12(1):14‐7. [MEDLINE: ] - PubMed
Kamar 2012 {published data only}
-
- Kamar N, Mariat C, Albano L, Villemain F, Moal MC, Ladriere M, et al. Improvement in renal function following conversion from low‐dose mycophenolate acid to higher‐dose enteric‐coated mycophenolate sodium (EC‐MPS) with concomitant tacrolimus reduction in maintenance kidney transplant recipients [abstract no: O‐295]. Transplant International 2009;22(Suppl 2):78.
-
- Kamar N, Rostaing L, Cassuto E, Villemain F, Moal MC, Ladriere M, et al. A multicenter, randomized trial of increased mycophenolic acid dose using enteric‐coated mycophenolate sodium with reduced tacrolimus exposure in maintenance kidney transplant recipients. Clinical Nephrology 2012;77(2):126‐36. [MEDLINE: ] - PubMed
Kandaswamy 2005 {published data only}
-
- Kandaswamy R, Humar A, Dunn T, Gross E, Hughes M, Hill M, et al. Prospective randomized trial of maintenance immunosuppression (IS) in prednisone (P)‐free recipients: 5‐year results [abstract no: 286]. American Journal of Transplantation 2008;8(Suppl 2):254. [CENTRAL: CN‐00677752]
-
- Kandaswamy R, Humar A, Khwaja K, Asolati M, Harmon J, Gillingham K, et al. A prospective randomized study of cyclosporine (CSA)/cellcept (MMF) vs. tacrolimus (TAC)/sirolimus (SIR) with rapid discontinuation of prednisone (P) [abstract]. American Journal of Transplantation 2003;3(Suppl 5):198. [CENTRAL: CN‐00445992]
-
- Kandaswamy R, Humar A, Sturdevant M, Garcia‐Roca R, Casingal V, Tan M, et al. Prospective randomized trial of maintenance immunosuppression (IS) in prednisone (P)‐free recipients: mixed results [abstract no: 329]. American Journal of Transplantation 2006;6(Suppl 2):178. [CENTRAL: CN‐00766092]
-
- Kandaswamy R, Humar A, Sutherland DE, Gillingham K, Matas A. A prospective, randomized study of cyclosporine (CSA)/mycophenolate mofetil (MMF) versus tacrolimus (TAC)/sirolimus(SIR) with rapid discontinuation of prednisone (P) [abstract no: 084]. Transplantation 2004;78(2 Suppl):32. [CENTRAL: CN‐00509261]
-
- Kandaswamy R, Melancon JK, Dunn T, Tan M, Casingal V, Humar A, et al. A prospective randomized trial of steroid‐free maintenance regimens in kidney transplant recipients‐‐an interim analysis. American Journal of Transplantation 2005;5(6):1529‐36. [MEDLINE: ] - PubMed
Keitel 1999 {published data only}
-
- Keitel E, Michelon T, Dominguez V, Bittar AE, Santos AF, Goldani JC, et al. Long‐term evaluation of two protocols of elective cyclosporine withdrawal in renal transplant recipients. Transplantation Proceedings 1999;31(7):3013‐5. [MEDLINE: ] - PubMed
Kovarik 2001 {published data only}
-
- Kaplan B, Polsky D, Weinfurt K, Fastenau J, Kim J, Ryu S, et al. Quality of life improvement and lower costs associated with simulect based induction therapy [abstract]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):733A. [CENTRAL: CN‐00401459]
-
- Kovarik JM, Pescovitz MD, Sollinger HW, Kaplan B, Legendre C, Salmela K, et al. Differential influence of azathioprine and mycophenolate mofetil on the disposition of basiliximab in renal transplant patients. Clinical Transplantation 2001;15(2):123‐30. [MEDLINE: ] - PubMed
-
- Pescovitz M, Kovarik JM, Gerbeau C, Simulect US‐O1 Study Group. Pharmacokinetics of basiliximab when coadministered with MMF in kidney transplantation [abstract no: 0112]. XVIII International Congress of the Transplantation Society; 2000 Aug 27‐Sep 1; Rome, Italy. 2000. [CENTRAL: CN‐00402225]
-
- Pescovitz MD, Barbeito R. Effect of "C2" cyclosporine levels and time to initiation of cyclosporine therapy on outcomes in patients receiving neoral and simulect [abstract no: A36]. Journal of the American Society of Nephrology 2000;11(Sept):703A. [CENTRAL: CN‐00433641]
-
- Pescovitz MD, Barbeito R, Simulect US 01 Study Group. Two‐hour post‐dose cyclosporine level is a better predictor than trough level of acute rejection of renal allografts. Clinical Transplantation 2002;16(5):378‐82. [MEDLINE: ] - PubMed
Kovarik 2003 {published data only}
-
- Kovarik JM, Dantal J, Civati G, Rizzo G, Rouilly M, Bettoni‐Ristic O, et al. Influence of delayed initiation of cyclosporine on everolimus pharmacokinetics in de novo renal transplant patients. American Journal of Transplantation 2003;3(12):1576‐80. [MEDLINE: ] - PubMed
Kovarik‐2306 2004 {published data only}
-
- Kovarik JM, Tedesco H, Pascual J, Civati G, Bizot MN, Geissler J, et al. Everolimus therapeutic concentration range defined from a prospective trial with reduced‐exposure cyclosporine in de novo kidney transplantation. Therapeutic Drug Monitoring 2004;26(5):499‐505. [MEDLINE: ] - PubMed
-
- Kovarik JM, Tedesco H, Pascual J, Civati G, Schmidli H, Geissler J. Everolimus therapeutic concentration range derived from prospective concentration‐controlled trial in de novo kidney transplantation [abstract]. American Journal of Transplantation 2004;4(Suppl 8):298. [CENTRAL: CN‐00509289] - PubMed
-
- Magee J, Tedesco H, Pascual J, Civati G, Filho G, Garcia V, et al. Efficacy and safety of 2 doses of everolimus combined with reduced dose neoral® in de novo kidney transplant recipients: 12 months analysis [abstract]. American Journal of Transplantation 2004;4(Suppl 8):296‐7. [CENTRAL: CN‐00509335]
-
- Pascual J, Cambi V, Dissegna D, Esmeraldo R, Lao M, Durlik M, et al. Efficacy and safety of 2 doses of everolimus combined with reduced dose Neoral® in de novo kidney transplant recipients: 24 months analysis [abstract no: 1010]. American Journal of Transplantation 2005;5(Suppl 11):414. [CENTRAL: CN‐00725012]
-
- Pascual J, Tedesco H, Civati G, Magee J, Haas T, Bernhardt P. Excellent renal function with concentration‐controlled everolimus, reduced exposure to neoral, and steroids in de novo kidney transplant recipients: results of a 6 months efficacy and safety analysis [abstract]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):786. [CENTRAL: CN‐00447112]
Liu 2002a {published data only}
-
- Liu J, Guan D. Chronic allograft nephropathy: a prospective randomized trial of cyclosporin reduction or changing tacrolimus with mycophenolate mofetil [abstract]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00416166]
Liu 2007b {published data only}
-
- Liu M, Zhang W, Gu M, Yin C, Zhang WY, Lv Q, et al. Protective effects of sirolimus by attenuating connective tissue growth factor expression in human chronic allograft nephropathy. Transplantation Proceedings 2007;39(5):1410‐5. [MEDLINE: ] - PubMed
Maiorano 2006 {published data only}
-
- Maiorano A, Stallone G, Schena A, Infante B, Pontrelli P, Schena FP, et al. Sirolimus interferes with iron homeostasis in renal transplant recipients. Transplantation 2006;82(7):908‐12. [MEDLINE: ] - PubMed
McGrath 2001 {published data only}
-
- McGrath JS, Shehata M. Chronic allograft nephropathy: prospective randomised trial of cyclosporin withdrawal and mycophenolate mofetil or tacrolimus substitution. Transplantation Proceedings 2001;33(3):2193‐5. [MEDLINE: ] - PubMed
-
- McGrath JS, Shehata M. Complete withdrawal of cyclosporin in chronic allograft nephropathy: a randomised prospective trial of conversion to mycophenolate mofetil or tacrolimus [abstract no: P0539]. XVIII International Congress of the Transplantation Society; 2000 Aug 27‐Sep 1; Rome, Italy. 2000. [CENTRAL: CN‐00433636]
McMaster 1983 {published data only}
-
- McMaster P, Haynes IG, Michael J. Cyclosporine in cadaveric renal transplantation: a prospective randomized trial. Transplantation Proceedings 1983;15(4 Suppl 1‐2):2523‐7. [EMBASE: 14164140]
Meier 2006 {published data only}
-
- Fricke L, Meier M, Mueller‐Steinhardt M, Ohltmann A, Jabs W, Steinhoff J, et al. Switching from cyclosporine to tacrolimus ‐ beneficial effects in patients with chronic allograft nephropathy over a 3 years period [abstract]. 3rd International Congress on Immunosuppression; 2004 Dec 8‐11; San Diego (CA). 2004.
-
- Meier M, Nitschke M, Weidtmann B, Jabs WJ, Wong W, Suefke S, et al. Slowing the progression of chronic allograft nephropathy by conversion from cyclosporine to tacrolimus: a randomized controlled trial. Transplantation 2006;81(7):1035‐40. [MEDLINE: ] - PubMed
Messa 2009 {published data only}
-
- Messa P, Alberti L, Montagnino G, Cafforio C, Berardinelli L, Rastaldi MP. T‐regulatory cell changes after early transition from tacrolimus to sirolimus in renal transplanted patients [abstract no: SU642]. World Congress of Nephrology; 2009 May 22‐26; Milan, Italy. 2009. [CENTRAL: CN‐00776834]
Metcalfe 2002 {published data only}
-
- Brook NR, Metcalf MS, Jain S, Bicknell GR, Nicholson ML, Harper SJ. A randomised trial of mycophenolate mofetil versus azathioprine as calcineurin inhibitor sparing agents in the treatment of chronic allograft nephropathy [abstract no: P‐97]. 3rd International Congress on Immunosuppression; 2004 Dec 8‐11; San Diego (CA). 2004. [CENTRAL: CN‐00550372] - PubMed
-
- Brook NR, Metcalfe MS, Waller JR, Jain S, Hosgood SA, Nicholson ML. A prospective randomised trial of mycophenolate mofetil and azathioprine after calcineurin reduction in renal allografts with established chronic allograft nephropathy [abstract]. American Journal of Transplantation 2004;4(Suppl 8):485. [CENTRAL: CN‐00509106]
-
- Metcalfe MS, Jain S, Waller JR, Saunders RN, Bicknell GR, Nicholson ML. A randomized trial of mycophenolate mofetil versus azathioprine as calcineurin inhibitor sparing agents in the treatment of chronic allograft nephropathy. Transplantation Proceedings 2002;34(5):1812‐4. [MEDLINE: ] - PubMed
Miserlis 2008 {published data only}
-
- Miserlis G, Papanikolaou V, Vergoulas G, Antoniadis N, Fouzas I, Vrochidis D, et al. Efficacy and safety of everolimus with low dose cyclosporine A compared with mycophenolate mofetil and full dose cyclosporine A in de novo renal transplant recipients [abstract no: 1641]. Transplantation 2008;86(Suppl 2):544. [CENTRAL: CN‐00740580]
Mourad 2004a {published data only}
-
- Mourad G, Rostaing L, Legendre C, Garrigue V, Thervet E, Durand D. Sequential protocols using basiliximab versus antithymocyte globulins in renal‐transplant patients receiving mycophenolate mofetil and steroids. Transplantation 2004;78(4):584‐90. [MEDLINE: ] - PubMed
-
- Mourad G, Rostaing L, Legendre C, Lorho R, Therver E, Fares N. Simulect versus thymoglobulin with delayed introduction of neoral in renal transplantation: three month results of a French multicenter randomized trial [abstract no: 0592]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00402018]
-
- Mourad GJ, Rostaing L, Legendre C, Garrigue V, Thervet E, Durand D. A sequential protocol using simulect vs thymoglobulin in low immunological risk renal transplant recipients: six‐month results of a French multicenter, randomized trial [abstract no: 1212]. American Journal of Transplantation 2003;3(Suppl 5):462. [CENTRAL: CN‐00446849]
Mourad 2005 {published data only}
-
- Budde K, Zeier M, Cohen D, Kirchherr B, MyProms SG. How much exposure is needed in the first week in patients receiving induction with basiliximab and enteric coated mycophenolate sodium? [abstract no: 1206]. American Journal of Transplantation 2005;5(Suppl 11):464.
-
- Kamar N, Garrigue V, Karras A, Mourad G, Lefrancois N, Charpentier B, et al. Impact of early or delayed cyclosporine on delayed graft function in renal transplant recipients: a randomized, multicenter study. American Journal of Transplantation 2006;6(5 Pt 1):1042‐8. [MEDLINE: ] - PubMed
-
- Legendre C, Cohen D, Zeier M, Rostaing L, Budde K. Efficacy and safety of enteric‐coated mycophenolate sodium in de novo renal transplant recipients: pooled data from three 12‐month multicenter, open‐label, prospective studies. Transplantation Proceedings 2007;39(5):1386‐91. [MEDLINE: ] - PubMed
-
- Legendre CH, Rostaing L, Kirchherr B, MyProms SG. Tolerability of enteric coated mycophenolate sodium (EC‐MPS) in combination with neoral and steroids in de novo kidney transplant recipients: a 12 months prospective trial [abstract no: 1209]. American Journal of Transplantation 2005;5(Suppl 11):464‐5.
-
- Mourad G, Karras A, Kamar N, Garrigue V, Legendre C, Lefrancois N, et al. Renal function with delayed or immediate cyclosporine microemulsion in combination with enteric‐coated mycophenolate sodium and steroids: results of follow up to 30 months post‐transplant. Clinical Transplantation 2007;21(3):295‐300. [MEDLINE: ] - PubMed
Mourer 2012 {published data only}
-
- Mourer JS, Ewe SH, Mallat MJ, Ng AC, Rabelink TJ, Bax JJ, et al. Late calcineurin inhibitor withdrawal prevents progressive left ventricular diastolic dysfunction in renal transplant recipients.[Erratum appears in Transplantation. 2013 Apr 15;95(7):e52]. Transplantation 2012;94(7):721‐8. [MEDLINE: ] - PubMed
-
- Mourer JS, Koning EJ, Zwet EW, Mallat MJ, Rabelink TJ, Fijter JW. Impact of late calcineurin inhibitor withdrawal on ambulatory blood pressure and carotid intima media thickness in renal transplant recipients. Transplantation 2013;96(1):49‐57. [MEDLINE: ] - PubMed
-
- Mourer JS, Koning EJP, Berger SP, Op't Roodt J, Spaans M, Rabelink AJ, et al. Effects of CNI or MMF withdrawal on carotid intima media thickness in renal transplant recipients [abstract no: 528]. American Journal of Transplantation 2008;8(Suppl 2):319.
-
- Mourer JS, Hartigh J, Mallat MJ, Berger SP. Estimation of systemic exposure improves safety of withdrawal of either CNI or MMF from triple drug therapy [abstract no: F‐PO628]. Journal of the American Society of Nephrology 2007;18(Abstracts):237A‐8A.
-
- Mourer JS, Hartigh J, Zwet EW, Mallat MJ, Dubbeld J, Fijter JW. Randomized trial comparing late concentration‐controlled calcineurin inhibitor or mycophenolate mofetil withdrawal. Transplantation 2012;93(9):887‐94. [MEDLINE: ] - PubMed
Noris 2007 {published data only}
-
- Noris M, Casiraghi F, Todeschini M, Cravedi P, Cugini D, Monteferrante G, et al. Regulatory T cells and T cell depletion: role of immunosuppressive drugs. Journal of the American Society of Nephrology 2007;18(3):1007‐18. [MEDLINE: ] - PubMed
-
- Ruggenenti P, Perico N, Gotti E, Cravedi P, D'Agati V, Gagliardini E, et al. Sirolimus versus cyclosporine therapy increases circulating regulatory T cells, but does not protect renal transplant patients given alemtuzumab induction from chronic allograft injury. Transplantation 2007;84(8):956‐64. [MEDLINE: ] - PubMed
-
- Todeschini M, Cortinovis M, Perico N, Poli F, Innocente A, Cavinato RA, et al. In kidney transplant patients, alemtuzumab but not basiliximab/low‐dose rabbit anti‐thymocyte globulin induces B cell depletion and regeneration, which associates with a high incidence of de novo donor‐specific anti‐HLA antibody development. Journal of Immunology 2013;191(5):2818‐28. [MEDLINE: ] - PubMed
Novoa 2011 {published data only}
-
- Grinyo JM, Paul J, Novoa P, Errasti P, Franco A, Aldana G, et al. Better renal function in renal‐transplant recipients treated with everolimus plus CSA elimination compared with CSA reduction [abstract no: P‐359]. Transplant International 2009;22(Suppl 2):183.
-
- Grinyo JM, Paul J, Novoa P, Errasti P, Franco A, Aldana G, et al. Better renal function in renal‐transplant recipients treated with everolimus plus cyclosporine elimination compared with cyclosporine minimisation [abstract no: 1636]. American Journal of Transplantation 2010;10(Suppl 4):503. [EMBASE: 70465011]
-
- Novoa PA, Grinyo JM, Ramos FJ, Errasti P, Franco A, Aldana G, et al. De novo use of everolimus with elimination or minimization of cyclosporine in renal transplant recipients. Transplantation Proceedings 2011;43(9):3331‐9. [MEDLINE: ] - PubMed
OPTIMA‐TX Study 2008 {published data only}
-
- Bolin P Jr, Shihab F, Mulloy L, Henning A, Gao J, Bartucci M, et al. Optimizing tacrolimus therapy in maintenance renal allografts: 6 month results [abstract no: 390]. American Journal of Transplantation 2006;6(Suppl 2):197‐8. [CENTRAL: CN‐00671805] - PubMed
-
- Bolin P Jr, Shihab FS, Mulloy L, Henning AK, Gao J, Bartucci M, et al. Optimizing tacrolimus therapy in the maintenance of renal allografts: 12‐month results. Transplantation 2008;86(1):88‐95. [MEDLINE: ] - PubMed
-
- Bolin P, Shihab F, Mulloy L, Gao J, Bartucci M, Holman J, et al. Optimizing tacrolimus therapy in maintenance renal allografts: 6 month results in African Americans (AA) [abstract no: F‐PO1077]. Journal of the American Society of Nephrology 2006;17(Abstracts):563A. [CENTRAL: CN‐00602098]
Pankewycz 2011 {published data only}
-
- Pankewycz O, Kohli R, Wallace PK, Said M, Feng L, Patel S, et al. Low dose rabbit anti‐thymocyte globulin induction therapy results in prolonged selective CD4 T cell depletion irrespective of maintenance immunosuppression [abstract no: 957]. American Journal of Transplantation 2010;10(Suppl 4):316‐7. [EMBASE: 70464333]
-
- Pankewycz O, Kohli R, Wallace PK, Said M, Feng L, Patel SK, et al. Low dose rabbit anti‐thymocyte globulin induction therapy results in prolonged selective CD4 T cell depletion irrespective of maintenance immunosuppression [abstract no: 130]. Transplantation 2010;90(Suppl S2):62. [EMBASE: 71531221] - PubMed
-
- Pankewycz O, Leca N, Kohli R, Wallace PK, Said M, Feng L, et al. Low‐dose rabbit antithymocyte globulin induction therapy results in prolonged selective lymphocyte depletion irrespective of maintenance immunosuppression. Transplantation Proceedings 2011;43(2):462‐5. [MEDLINE: ] - PubMed
-
- Pankewycz O, Leca N, Kohli R, Weber‐Shrikant E, Said M, Alnimri M, et al. Conversion to low‐dose tacrolimus or rapamycin 3 months after kidney transplantation: a prospective, protocol biopsy‐guided study. Transplantation Proceedings 2011;43(2):519‐23. [MEDLINE: ] - PubMed
-
- Pankewycz O, Leca N, Said M, Feng L, Patel S, Kohli R, et al. A protocol biopsy directed randomized trial comparing tacrolimus minimization to sirolimus conversion at 3 months results in an equivalent degree of histological injury at 1 year and equivalent renal function at 2 years [abstract no: 948]. American Journal of Transplantation 2012;12(Suppl 3):304. [EMBASE: 70746901]
Ponticelli 1988 {published data only}
-
- Ponticelli C, Tarantino A, et al. Prospective trial of triple therapy in renal transplants [abstract]. Nephrology Dialysis Transplantation 1988;3(1):98. [CENTRAL: CN‐00260367]
Rahamimov 2008 {published data only}
-
- Rahamimov R, Yusim A, Winkler J, Mashraki T, Gafter U, Mor E. Conversion from tacrolimus to sirolimus‐based protocol in renal transplant recipients: a long‐term comparative study [abstract no: 1617]. Transplantation 2008;86(Suppl 2):537. [CENTRAL: CN‐00740447]
Ritz 1998 {published data only}
-
- Kahn D, Botha JF, Pascoe MD, Pontin AR, Halkett J, Tandon V. Withdrawal of cyclosporine in renal transplant recipients with acute tubular necrosis improves renal function. Transplant International 2000;13(Suppl 1):S82‐3. [MEDLINE: ] - PubMed
-
- Ritz M, Pascoe MD, Pontin AR, Kahn D. Is cyclosporine toxic to the transplanted kidney with acute tubular necrosis?. Transplantation Proceedings 1998;30(4):1204. [MEDLINE: ] - PubMed
Saunders 2003 {published data only}
-
- Saunders RN, Bicknell GR, Nicholson ML. The impact of cyclosporine dose reduction with or without the addition of rapamycin on functional, molecular, and histological markers of chronic allograft nephropathy. Transplantation 2003;75(6):772‐80. [MEDLINE: ] - PubMed
-
- Saunders RN, Carr S, Nicholson ML. Chronic allograft nephropathy: a prospective randomised trial of cyclosporin reduction with or without rapamycin [abstract]. Transplantation 2000;69(8 Suppl):S226. [CENTRAL: CN‐00447600]
-
- Saunders RN, Carr S, Nicholson ML. Chronic allograft nephropathy: a prospective randomized trial of cyclosporin reduction with or without rapamycin [abstract]. British Journal of Surgery 2000;87 Suppl 1:44.
-
- Saunders RN, Carr S, Nicholson ML. Side‐effect profile of rapamycin in patients with chronic allograft nephropathy [abstract]. British Journal of Surgery 2000;87 Suppl 1:82. [CENTRAL: CN‐00339343]
SOCRATES Study 2014 {published data only}
-
- Russ G, Eris J, Kanellis J, Hutchison B, Hibberd A, Pilmore H, et al. Multicentre RCT of early switch to everolimus plus steroids or everolimus plus CSA versus CSA, MPA and steroids in de novo kidney transplant recipients: 12 month analysis [abstract no: 93]. Transplantation Society of Australia & New Zealand (TSANZ). 30th Annual Scientific Meeting; 2012 Jun 27‐29; Canberra (ACT). 2012:103.
Westhoff 1995 {published data only}
-
- Westhoff A, Heering P, Ivens K, Kutkuhn B, Grabensee B. Safe immunosuppression after kidney transplantation even without cyclosporine? A prospective, randomised study following primary triple therapy [Sichere immunsuppression nach nierentransplantation auch ohne ciclosporin? eine prospektive, randomisierte untersuchung nach primarer triple‐therapie]. Transplantationsmedizin ‐ Organ Der Deutschen Transplantationsgesellschaft 1995;7(1):27‐32. [EMBASE: 1995161557]
Wu 2007d {published data only}
-
- Wu MJ, Shu KH, Cheng CH, Chen CH, Yu DM. Conversion from cyclosporine to sirolimus in stable Taiwanese kidney transplant recipients ‐ one year report [abstract no: P442]. Transplant International 2007;20(Suppl 2):201.
References to ongoing studies
David‐Neto 2014 {published data only}
-
- David‐Neto E, Galante N, Altona M, Paula F, Triboni A, Ramos F, et al. A randomized, prospective study comparing everolimus/low tacrolimus with regular tacrolimus/MPS for the elderly renal transplant recipients [abstract]. Transplantation 2014;98(Suppl 1):549. [EMBASE: 71545379]
ERIC Study 2010 {published data only}
-
- Sanchez‐Fructuoso A, Ruiz JC, Hernandez D, Sanchez‐Plumed J, Fernandez A, Pastor Rodriguez A, et al. Early everolimus introduction and calcineurin inhibitor withdrawal in renal transplant patients: a multicenter, randomized, open‐label study (the ERIC study) [abstract no: 1647]. American Journal of Transplantation 2010;10(Suppl 4):506. [EMBASE: 70465022]
ISRCTN63298320 {published data only}
-
- Nicholson M. A prospective randomised trial of the use of cellcept to allow early tacrolimus withdrawal in live donor kidney transplantation. www.isrctn.com/ISRCTN63298320 (accessed 6 February 2017).
TRANSFORM Study 2013 {published data only}
-
- Chadban S, Hughes P, Campbell S, Irish A, Lim W, O'Connell P, et al. TRANSFORM trial design: a randomized, multicentre, open‐label study of everolimus with reduced calcineurin inhibitors in over 20000 de novo renal transplant recipients [abstract no:48]. Transplantation Society of Australia & New Zealand (TSANZ). 32nd Annual Scientific Meeting; 2014 Jun 11‐13; Canberra (ACT). 2014:61.
-
- Legendre C, Srinivas T, Pascual J, Chadban S, Citterio F, Henry M, et al. The TRANSFORM trial design: a large randomized, multicenter, open‐label study of everolimus with reduced calcineurin inhibitors in de novo renal transplantation [abstract no: P16]. Transplant International 2013;26(Suppl 3):23‐4. [EMBASE: 71356170]
-
- Pascual J, Chadban S, Citterio F, Henry M, Legendre C, Oppenheimer F, et al. TRANSFORM trial design: effect of everolimus on long‐term outcomes after kidney transplantation [abstract no: P308]. Transplant International 2013;26(Suppl 2):247. [EMBASE: 71359928]
-
- Pascual J, Srinivas TR, Chadban S, Citterio F, Henry M, Legendre C, et al. Balancing efficacy and renal function preservation after kidney transplantation with everolimus and reduced calcineurin inhibitors for better graft outcomes: design of the TRANSFORM study [abstract]. Nephrology Dialysis Transplantation 2014;29(Suppl 3):iii535. [EMBASE: 71493013]
-
- Pascual J, Srinivas TR, Chadban S, Citterio F, Oppenheimer F, Tedesco H, et al. TRANSFORM: a novel study design to evaluate the effect of everolimus on long‐term outcomes after kidney transplantation. Open Access Journal of Clinical Trials 2013;6:45‐54. [EMBASE: 605631666]
Additional references
Ahsan 2001
-
- Ahsan N, Johnson C, Gonwa T, Halloran P, Stegall M, Hardy M, et al. Randomized trial of tacrolimus plus mycophenolate mofetil or azathioprine versus cyclosporine oral solution (modified) plus mycophenolate mofetil after cadaveric kidney transplantation: results at 2 years. Transplantation 2001;72(2):245‐50. [MEDLINE: ] - PubMed
Bennett 1996
-
- Bennett WM, DeMattos A, Meyer MM, Andoh T, Barry JM. Chronic cyclosporine nephropathy: the Achilles' heel of immunosuppressive therapy. Kidney International 1996;50(4):1089‐100. [MEDLINE: ] - PubMed
Gonwa 2002
-
- Gonwa TA, Hricik DE, Brinker K, Grinyo JM, Schena FP, Sirolimus Renal Function Study Group. Improved renal function in sirolimus‐treated renal transplant patients after early cyclosporine elimination. Transplantation 2002;74(11):1560‐7. [MEDLINE: ] - PubMed
GRADE 2008
Hariharan 2000
-
- Hariharan S, Johnson CP, Bresnahan BA, Taranto SE, McIntosh MJ, Stablein D. Improved graft survival after renal transplantation in the United States, 1988 to 1996. New England Journal of Medicine 2000;342(9):605‐12. [MEDLINE: ] - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kasiske 1996
-
- Kasiske BL, Guijarro C, Massy ZA, Wiederkehr MR, Ma JZ. Cardiovascular disease after renal transplantation. Journal of the American Society of Nephrology 1996;7(1):158‐65. [MEDLINE: ] - PubMed
Lim 2014
-
- Lim W.H, Eris J, Kannellis J, Pussell B, Wild Z, Witcombe D, et al. A systematic review of conversion from calcineurin inhibitor to mammalian target of rapamycin inhibitors for maintenance immunosuppression in kidney transplant recipients. American Journal of Transplantation 2014;14(9):2106‐19. [MEDLINE: ] - PubMed
Mason 2014
Melk 2003
-
- Melk A, Halloran P. Immunosuppressive agents used in transplantation. In: Johnson RJ, Feehally J editor(s). Comprehensive Clinical Nephrology. 2nd Edition. Mosby, 2003:1062‐4.
Moore 2009
-
- Moore J, Middleton L, Cockwell P, Adu D, Ball S, Little MA, et al. Calcineurin Inhibitor sparing with mycophenolate in kidney transplantation: A systematic review and meta‐analysis. Transplantation 2009;87(4):591‐605. [MEDLINE: ] - PubMed
Schünemann 2011a
-
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Schünemann 2011b
-
- Schünemann HJ, Oxman AD, Higgins JP, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

