Salmonella Intracellular Lifestyles and Their Impact on Host-to-Host Transmission
- PMID: 28730976
- PMCID: PMC11687531
- DOI: 10.1128/microbiolspec.MTBP-0009-2016
Salmonella Intracellular Lifestyles and Their Impact on Host-to-Host Transmission
Abstract
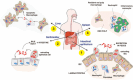
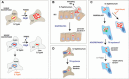
More than a century ago, infections by Salmonella were already associated with foodborne enteric diseases with high morbidity in humans and cattle. Intestinal inflammation and diarrhea are hallmarks of infections caused by nontyphoidal Salmonella serovars, and these pathologies facilitate pathogen transmission to the environment. In those early times, physicians and microbiologists also realized that typhoid and paratyphoid fever caused by some Salmonella serovars could be transmitted by "carriers," individuals outwardly healthy or at most suffering from some minor chronic complaint. In his pioneering study of the nontyphoidal serovar Typhimurium in 1967, Takeuchi published the first images of intracellular bacteria enclosed by membrane-bound vacuoles in the initial stages of the intestinal epithelium penetration. These compartments, called Salmonella-containing vacuoles, are highly dynamic phagosomes with differing biogenesis depending on the host cell type. Single-cell studies involving real-time imaging and gene expression profiling, together with new approaches based on genetic reporters sensitive to growth rate, have uncovered unprecedented heterogeneous responses in intracellular bacteria. Subpopulations of intracellular bacteria displaying fast, reduced, or no growth, as well as cytosolic and intravacuolar bacteria, have been reported in both in vitro and in vivo infection models. Recent investigations, most of them focused on the serovar Typhimurium, point to the selection of persisting bacteria inside macrophages or following an autophagy attack in fibroblasts. Here, we discuss these heterogeneous intracellular lifestyles and speculate on how these disparate behaviors may impact host-to-host transmissibility of Salmonella serovars.
Keywords: Salmonella-containing vacuole; autophagy; carrier state; cytosolic bacteria; extracellular; intracellular lifestyle; persistence; transmission.
Figures
References
-
- Wiesner M, Calva JJ, Bustamante VH, Pérez-Morales D, Fernández-Mora M, Calva E, Silva C. 2016. A multi-drug resistant Salmonella Typhimurium ST213 human-invasive strain (33676) containing the blaCMY-2 gene on an IncF plasmid is attenuated for virulence in BALB/c mice. BMC Microbiol 16:18. 10.1186/s12866-016-0633-7. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

