An illustrated guide for characters and terminology used in descriptions of Phlebotominae (Diptera, Psychodidae)
- PMID: 28730992
- PMCID: PMC5520390
- DOI: 10.1051/parasite/2017027
An illustrated guide for characters and terminology used in descriptions of Phlebotominae (Diptera, Psychodidae)
Abstract
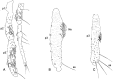
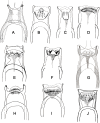
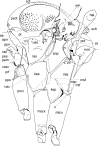
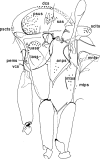
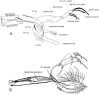
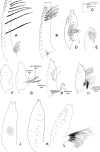
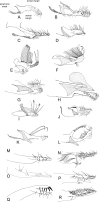
Phlebotomine (Diptera, Psychodidae, Phlebotominae) taxonomy has been studied extensively, primarily due to the role of these flies as vectors of various parasites, including species of Leishmania, Bartonella and arboviruses that cause diseases in humans and other vertebrates. We present some topics discussed at a round-table on phlebotomine taxonomy held at the Ninth International Symposium on Phlebotomine Sandflies (ISOPS IX) in Reims, France, in June 2016. To date, approximately one thousand phlebotomine species have been described worldwide, although in varying languages and mostly without standardization of characters and terminology. In the interest of standardization, we list the characters that should minimally be considered in the description of new phlebotomine taxa as well as annotated illustrations of several characters. For these characters, multiple illustrations are provided to show some of the variations. The preferred terms for all pertinent characters are listed as well as their synonyms in English, Portuguese, and French. Finally, we offer an updated list of abbreviations to be used for generic and subgeneric names.
La taxonomie des phlébotomes (Diptera, Psychodidae, Phlebotominae) a été largement étudiée, principalement en raison du rôle de ces diptères comme vecteurs de divers parasites, y compris des espèces de Leishmania, Bartonella et des arbovirus qui causent des maladies chez l’homme et d’autres vertébrés. Nous présentons certains thèmes abordés lors d’une table ronde sur la taxonomie des phlébotomes tenue lors du 9ème Symposium international sur les phlébotomes (ISOPS IX) à Reims, France, en juin 2016. À ce jour, environ mille espèces de phlébotomes ont été décrites dans le monde entier, bien que dans des langues variées et surtout sans standardisation des caractères et de la terminologie. Dans l’intérêt de la normalisation, nous énumérons les caractères qui devraient être considérés lors de la description de nouveaux taxons de phlébotomes et fournissons des illustrations légendées de nombre d’entre eux. Pour ces caractères, plusieurs illustrations sont fournies pour montrer une partie de la variabilité. Les termes préférés pour tous les caractères pertinents sont répertoriés ainsi que leurs synonymes en anglais, portugais et français. Enfin, nous proposons une liste actualisée des abréviations à utiliser pour les genres et sous-genres.
© E.A.B. Galati et al., published by EDP Sciences, 2017.
Figures









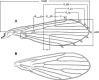

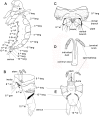
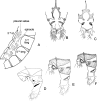
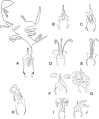
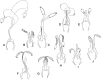



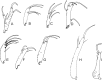


References
-
- Croset H, Abonnenc E, Rioux J. 1970. Phlebotomus (Paraphlebotomus) chabaudi n. sp. (Diptera-Psychodidae). Annales de Parasitologie Humaine et Comparée, 45, 863–873. - PubMed
-
- Cumming JM, Wood DM. 2009. Adult morphology and terminology, in Manual of Central American Diptera, vol. 1, Brown BV, Borkent A, Cumming JM, Wood DM, Woodley NE, Zumbado MA, Editors National Research Council of Ottawa: Canada: p. 9–502.
-
- Galati EAB. 2003. Classificação de Phlebotominae, in Flebotomíneos do Brasil. Rangel EF, Lainson R, Editors Ed. Fiocruz: Rio de Janeiro: p. 23–51.
-
- Galati EAB. 2016. Phlebotominae (Diptera, Psychodidae): Classificação, morfologia, terminologia e identificação de Adultos, Apostila – Disciplina PSP5127-1 Bioecologia e Identificação de Phlebotominae. Public Heath School. University of São Paulo, www.fsp.usp.br/~egalati.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
