Molecular genetics and targeted therapy of WNT-related human diseases (Review)
- PMID: 28731148
- PMCID: PMC5547940
- DOI: 10.3892/ijmm.2017.3071
Molecular genetics and targeted therapy of WNT-related human diseases (Review)
Abstract
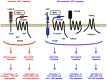
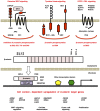
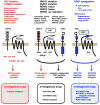
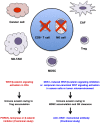
Canonical WNT signaling through Frizzled and LRP5/6 receptors is transduced to the WNT/β-catenin and WNT/stabilization of proteins (STOP) signaling cascades to regulate cell fate and proliferation, whereas non-canonical WNT signaling through Frizzled or ROR receptors is transduced to the WNT/planar cell polarity (PCP), WNT/G protein-coupled receptor (GPCR) and WNT/receptor tyrosine kinase (RTK) signaling cascades to regulate cytoskeletal dynamics and directional cell movement. WNT/β-catenin signaling cascade crosstalks with RTK/SRK and GPCR-cAMP-PKA signaling cascades to regulate β-catenin phosphorylation and β-catenin-dependent transcription. Germline mutations in WNT signaling molecules cause hereditary colorectal cancer, bone diseases, exudative vitreoretinopathy, intellectual disability syndrome and PCP-related diseases. APC or CTNNB1 mutations in colorectal, endometrial and prostate cancers activate the WNT/β-catenin signaling cascade. RNF43, ZNRF3, RSPO2 or RSPO3 alterations in breast, colorectal, gastric, pancreatic and other cancers activate the WNT/β-catenin, WNT/STOP and other WNT signaling cascades. ROR1 upregulation in B-cell leukemia and solid tumors and ROR2 upregulation in melanoma induce invasion, metastasis and therapeutic resistance through Rho-ROCK, Rac-JNK, PI3K-AKT and YAP signaling activation. WNT signaling in cancer, stromal and immune cells dynamically orchestrate immune evasion and antitumor immunity in a cell context-dependent manner. Porcupine (PORCN), RSPO3, WNT2B, FZD5, FZD10, ROR1, tankyrase and β-catenin are targets of anti-WNT signaling therapy, and ETC-159, LGK974, OMP-18R5 (vantictumab), OMP-54F28 (ipafricept), OMP-131R10 (rosmantuzumab), PRI-724 and UC-961 (cirmtuzumab) are in clinical trials for cancer patients. Different classes of anti-WNT signaling therapeutics are necessary for the treatment of APC/CTNNB1-, RNF43/ZNRF3/RSPO2/RSPO3- and ROR1-types of human cancers. By contrast, Dickkopf-related protein 1 (DKK1), SOST and glycogen synthase kinase 3β (GSK3β) are targets of pro-WNT signaling therapy, and anti-DKK1 (BHQ880 and DKN-01) and anti-SOST (blosozumab, BPS804 and romosozumab) monoclonal antibodies are being tested in clinical trials for cancer patients and osteoporotic post-menopausal women. WNT-targeting therapeutics have also been applied as reagents for in vitro stem-cell processing in the field of regenerative medicine.
Figures

References
-
- Katoh M. WNT and FGF gene clusters (Review) Int J Oncol. 2002;21:1269–1273. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

