The role of T helper 17 cells in the pathogenesis of hepatitis B virus-related liver cirrhosis (Review)
- PMID: 28731149
- PMCID: PMC5646947
- DOI: 10.3892/mmr.2017.7044
The role of T helper 17 cells in the pathogenesis of hepatitis B virus-related liver cirrhosis (Review)
Abstract
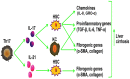
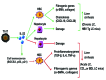
In chronic hepatitis B virus (HBV)-infected patients, T helper 17 (Th17) cells are significantly elevated. Th17 cells initiate immune‑mediated pathogenesis and have a critical role in the process of HBV‑related liver cirrhosis (HBV‑LC). The mechanisms underlying this process are attributed to Th17‑secreted cytokines, which include interleukin (IL)‑17, IL‑21 and IL‑22; however, a systemic analysis regarding these mechanisms has yet to be conducted. Therefore, the present study aimed to investigate the role of Th17 cells in the pathogenesis of HBV‑LC. All randomized clinical trials, case series, case reports and meta‑analyses that contained the aforementioned keywords were included in the review process. In addition, unpublished information from the Food and Drug Administration was included. The findings indicated that Th17‑secreted cytokines, including IL‑17, IL‑21 and IL‑22, function by activating or silencing hepatic stellate cells, modulating proinflammatory and pro‑ or antifibrogenic effectors, regulating extracellular matrix formation, upregulating chemokine expression, and inducing hepatocellular damage or hepatoprotection during the HBV‑LC process. In addition, Th17 cells and Th17‑secreted cytokines may be considered a potential tool in the diagnosis or treatment of HBV‑LC. The present review summarized the role of Th17 cells in the pathogenesis of HBV‑LC in order to deepen the clinical understanding of the role of Th17 cells and also to support the development of effective therapies for patients with HBV‑LC.
Figures
Similar articles
-
Ratios of regulatory T cells/T-helper 17 cells and transforming growth factor-β1/interleukin-17 to be associated with the development of hepatitis B virus-associated liver cirrhosis.J Gastroenterol Hepatol. 2014 May;29(5):1065-72. doi: 10.1111/jgh.12459. J Gastroenterol Hepatol. 2014. PMID: 24236690
-
Levels of hepatic Th17 cells and regulatory T cells upregulated by hepatic stellate cells in advanced HBV-related liver fibrosis.J Transl Med. 2017 Apr 11;15(1):75. doi: 10.1186/s12967-017-1167-y. J Transl Med. 2017. PMID: 28399886 Free PMC article.
-
Restoring homeostasis of CD4⁺ T cells in hepatitis-B-virus-related liver fibrosis.World J Gastroenterol. 2015 Oct 14;21(38):10721-31. doi: 10.3748/wjg.v21.i38.10721. World J Gastroenterol. 2015. PMID: 26478664 Free PMC article. Review.
-
Increased Th17 cells contribute to disease progression in patients with HBV-associated liver cirrhosis.J Viral Hepat. 2012 Jun;19(6):396-403. doi: 10.1111/j.1365-2893.2011.01561.x. Epub 2012 Mar 28. J Viral Hepat. 2012. PMID: 22571901
-
Pivotal roles of the interleukin-23/T helper 17 cell axis in hepatitis B.Liver Int. 2012 Jul;32(6):894-901. doi: 10.1111/j.1478-3231.2012.02764.x. Epub 2012 Feb 19. Liver Int. 2012. PMID: 22340646 Review.
Cited by
-
Cytokines and Chemokines in HBV Infection.Front Mol Biosci. 2021 Dec 2;8:805625. doi: 10.3389/fmolb.2021.805625. eCollection 2021. Front Mol Biosci. 2021. PMID: 34926586 Free PMC article. Review.
-
Hepatitis Virus Reactivation in Patients with Psoriasis Treated with Secukinumab in a Real-World Setting of Hepatitis B or Hepatitis C Infection.Clin Drug Investig. 2022 Jun;42(6):525-531. doi: 10.1007/s40261-022-01163-5. Epub 2022 May 28. Clin Drug Investig. 2022. PMID: 35633470 Free PMC article.
-
Interleukin-21 in Viral Infections.Int J Mol Sci. 2021 Sep 1;22(17):9521. doi: 10.3390/ijms22179521. Int J Mol Sci. 2021. PMID: 34502427 Free PMC article. Review.
-
Infection Risk and Vaccination in the Management of Psoriasis: Considerations for Biologic Therapy.Psoriasis (Auckl). 2025 Apr 11;15:127-144. doi: 10.2147/PTT.S510141. eCollection 2025. Psoriasis (Auckl). 2025. PMID: 40237012 Free PMC article. Review.
-
[Effects of hepatitis B virus on Th17, Treg and Th17/Treg ratio in different alanine aminetransferase stages].Beijing Da Xue Xue Bao Yi Xue Ban. 2022 Apr 18;54(2):272-277. doi: 10.19723/j.issn.1671-167X.2022.02.012. Beijing Da Xue Xue Bao Yi Xue Ban. 2022. PMID: 35435191 Free PMC article. Chinese.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

