Polyclonal and monoclonal antibodies for treating acute rejection episodes in kidney transplant recipients
- PMID: 28731207
- PMCID: PMC6483358
- DOI: 10.1002/14651858.CD004756.pub4
Polyclonal and monoclonal antibodies for treating acute rejection episodes in kidney transplant recipients
Abstract
Background: Registry data shows that the incidence of acute rejection has been steadily falling. Approximately 10% to 35% of kidney recipients will undergo treatment for at least one episode of acute rejection within the first post-transplant year. Treatment options include pulsed steroid therapy, the use of an antibody preparation, the alteration of background immunosuppression, or combinations of these options. Over recent years, new treatment strategies have evolved, and in many parts of the world there has been an increase in use of tacrolimus and mycophenolate and a reduction in the use of cyclosporin and azathioprine use as baseline immunosuppression to prevent acute rejection. There are also global variations in use of polyclonal and monoclonal antibodies to treat acute rejection. This is an update of a review published in 2006.
Objectives: The aim of this systematic review was to: (1) to evaluate the relative and absolute effects of different classes of antibody preparation in preventing graft loss and resolving cellular or humoral rejection episodes when used as a treatment for first episode of rejection in kidney transplant recipients; (2) evaluate the relative and absolute effects of different classes of antibody preparation in preventing graft loss and resolving cellular or humoral rejection episodes when used as a treatment for steroid-resistant rejection in kidney transplant recipients; (3) determine how the benefits and adverse events vary for each type of antibody preparation; and (4) determine how the benefits and harms vary for different formulations of antibody within each type.
Search methods: We searched the Cochrane Kidney and Transplant Specialised Register to 18 April 2017 through contact with the Information Specialist using search terms relevant to this review.
Selection criteria: Randomised controlled trials (RCTs) in all languages comparing all mono- and polyclonal antibody preparations, given in combination with any other immunosuppressive agents, for the treatment of cellular or humoral graft rejection, when compared to any other treatment for acute rejection were eligible for inclusion.
Data collection and analysis: Two authors independently assessed the risk of bias of the included studies and extracted data. Statistical analyses were performed using a random-effects model and results expressed as risk ratio (RR) or mean difference (MD) with 95% confidence intervals (CI).
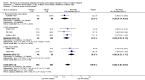
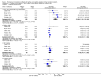
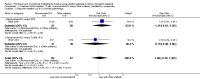
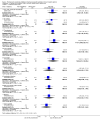
Main results: We included 11 new studies (18 reports, 346 participants) in this update, bring the total number of included studies to 31 (76 reports, 1680 participants). Studies were generally small, incompletely reported, especially for potential harms, and did not define outcome measures adequately. The risk of bias was inadequate or unclear risk for random sequence generation (81%), allocation concealment (87%) and other bias (87%). There were, however, a predominance of low risk of bias for blinding (75%) and incomplete outcome data (80%) across all the studies. Selective reporting had a mixture of low (58%), high (29%), and unclear (13%) risk of bias.Seventeen studies (1005 participants) compared therapies for first acute cellular rejection episodes. Antibody therapy was probably better than steroid in reversing acute cellular rejection (RR 0.50, 95% CI 0.30 to 0.82; moderate certainty) and preventing subsequent rejection (RR 0.70, 95% CI 0.50 to 0.99; moderate certainty), may be better for preventing graft loss (death censored: (RR 0.80, 95% CI 0.57 to 1.12; low certainty) but there was little or no difference in death at one year. Adverse effects of treatment (including fever, chills and malaise following drug administration) were probably reduced with steroid therapy (RR 23.88, 95% CI 5.10 to 111.86; I2 = 16%; moderate certainty).Twelve studies (576 patients) investigated antibody treatment for steroid-resistant rejection. There was little or no benefit of muromonab-CD3 over ATG or ALG in reversing rejection, preventing subsequent rejection, or preventing graft loss or death. Two studies compared the use of rituximab for treatment of acute humoral rejection (58 patients). Muromonab-CD3 treated patients suffered three times more than those receiving either ATG or T10B9, from a syndrome of fever, chills and malaise following drug administration (RR 3.12, 95% CI 1.87 to 5.21; I2 = 31%), and experienced more neurological side effects (RR 13.10 95% CI 1.43 to 120.05; I2 = 36%) (low certainty evidence).There was no evidence of additional benefit from rituximab in terms of either reversal of rejection (RR 0.94, 95% CI 0.54 to 1.64), or graft loss or death 12 months (RR 1.0, 95% CI 0.23 to 4.35). Rituximab plus steroids probably increases the risk of urinary tract infection/pyelonephritis (RR 5.73, 95% CI 1.80 to 18.21).
Authors' conclusions: In reversing first acute cellular rejection and preventing graft loss, any antibody is probably better than steroid, but there is little or no difference in subsequent rejection and patient survival. In reversing steroid-resistant rejection there was little or no difference between different antibodies over a period of 12 months, with limited data beyond that time frame. In treating acute humoral rejection, there was no evidence that the use of antibody therapy conferred additional benefit in terms of reversal of rejection, or death or graft loss.Although this is an updated review, the majority of newer included studies provide additional evidence from the cyclosporin/azathioprine era of kidney transplantation and therefore conclusions cannot necessarily be extrapolated to patients treated with more contemporary immunosuppressive regimens which include tacrolimus/mycophenolate or sirolimus. However, many kidney transplant centres around the world continue to use older immunosuppressive regimes and the findings of this review remain strongly relevant to their clinical practice.Larger studies with standardised reproducible outcome criteria are needed to investigate the outcomes and risks of antibody treatments for acute rejection in kidney transplant recipients receiving contemporary immunosuppressive regimes.
Conflict of interest statement
AW, SW, KT, MP SC: no conflicts to declare.
JRC
Figures



























Update of
-
Polyclonal and monoclonal antibodies for treating acute rejection episodes in kidney transplant recipients.Cochrane Database Syst Rev. 2006 Apr 19;(2):CD004756. doi: 10.1002/14651858.CD004756.pub3. Cochrane Database Syst Rev. 2006. Update in: Cochrane Database Syst Rev. 2017 Jul 20;7:CD004756. doi: 10.1002/14651858.CD004756.pub4. PMID: 16625610 Updated.
References
References to studies included in this review
Alamartine 1994 {published and unpublished data}
-
- Alamartine E, Bellakoul R, Berthoux F. Randomized prospective study comparing OKT3 and antithymocyte globulins for treatment of the first acute cellular rejection of kidney allografts. Transplantation Proceedings 1994;26(1):273‐4. [MEDLINE: ] - PubMed
Baldi 2000 {published data only}
-
- Baldi A, Malaise J, Mourad M, Squifflet JP. A prospective randomized study comparing poly‐ATG to mono‐OKT3 clonal antibodies for the first rejection therapy after kidney transplantation: long‐term results. Transplantation Proceedings 2000;32(2):429‐31. [MEDLINE: ] - PubMed
Birkeland 1975 {published and unpublished data}
-
- Birkeland SA. A controlled clinical trial of treatment with ALG in established rejection of renal allografts. Acta Medica Scandinavica 1975;198(6):489‐96. [MEDLINE: ] - PubMed
-
- Birkeland SA. The use of antilymphocyte globulin in renal allograft rejection. A controlled study. Postgraduate Medical Journal 1976;52(5 Suppl):82‐8. [MEDLINE: ] - PubMed
Blumke 1989 {published data only}
-
- Blumke M, Kirste G, Wanner U, Wilms H. Single center randomized trial using ATG v OKT3 treatment in steroid resistant rejection crises after kidney transplantation. Transplantation Proceedings 1989;21(1 Pt 2):1747. [MEDLINE: ] - PubMed
Broyer 1987a {published data only}
-
- Broyer M, Niaudet P, Bijaoui M, Gagnadoux MF. Treatment of acute rejection crisis by antilymphocyte globulins: a randomized prospective study in pediatric kidney transplantation. Transplantation Proceedings 1987;19(1 Pt 3):1886‐8. [MEDLINE: ] - PubMed
Campistol 1990 {published data only}
-
- Campistol JM, Oppenheimer F, Vilardell J, Ricart MJ, Andreu J. Randomized trial between OK‐T3 monoclonal antibody and antilymphocyte globulin (ALG) for acute vascular graft rejection (AVGR) [abstract]. 11th International Congress of Nephrology; 1990 Jul 15‐20; Tokyo, Japan. 1990:514A. [CENTRAL: CN‐00716031]
-
- Poch E, Oppenheimer F, Darnell A, Campistol JM, Andreu J, Lopez‐Pedret J. A randomized prospective comparison of ALG with OKT3 for treatment of renal vascular rejection [abstract]. Nephrology Dialysis Transplantation 1992;7(7):785.
Casadei 1998 {published data only}
-
- Casadei D, Rial M, Argento J, Goldberg J, Raimondi E. Preliminary results from a randomized and prospective study about immunoglobulin (IVIg) high doses vs. MoAb in the rescue of steroid resistant rejections [abstract]. Journal of the American Society of Nephrology 1997;8(Program & Abstracts):677. [CENTRAL: CN‐00444696]
-
- Casadei D, Rial M, Argento J, Goldberg J, Raimondi E. Preliminary results from a randomized and prospective study of high‐dose immunoglobulin versus monoclonal antibody in the rescue of steroid‐resistant rejections. Transplantation Proceedings 1998;30(5):2164. [MEDLINE: ] - PubMed
-
- Casadei DH, C Rial M, Opelz G, Golberg JC, Argento JA, Greco G, et al. A randomized and prospective study comparing treatment with high‐dose intravenous immunoglobulin with monoclonal antibodies for rescue of kidney grafts with steroid‐resistant rejection. Transplantation 2001;71(1):53‐8. [MEDLINE: ] - PubMed
Filo 1980 {published data only}
-
- Filo RS, Smith EJ, Leapman SB. Reversal of acute renal allograft rejection with adjunctive AG therapy. Transplantation Proceedings 1981;13(1 Pt 1):482‐90. [MEDLINE: ] - PubMed
-
- Filo RS, Smith EJ, Leapman SB. Therapy of acute cadaveric renal allograft rejection with adjunctive antithymocyte globulin. Transplantation 1980;30(6):445‐9. [MEDLINE: ] - PubMed
Gaber 1998 {published data only}
-
- First MR, US Multicenter Thymoglobulin Study Group. Thymoglobulin successfully prevents recurrent rejection [abstract]. 16th Annual Meeting. American Society of Transplant Physicians (ASTP); 1997 May 10‐14; Chicago (ILL). 1997:259. [CENTRAL: CN‐00509191]
-
- Gaber AO, First MR, Tesi RJ, Gaston RS, Mendez R, Mulloy LL, et al. Results of the double‐blind, randomized, multicenter, phase III clinical trial of Thymoglobulin versus ATGAM in the treatment of acute graft rejection episodes after renal transplantation. Transplantation 1998;66(1):29‐37. [MEDLINE: ] - PubMed
-
- Gaber AO, US Multicenter Thymoglobulin Study Group. The 1996 double blinded randomized multicenter phase iii clinical trial of thymoglobulin versus ATGAM in the treatment of acute graft rejection following renal transplantation [abstract]. 16th Annual Meeting. American Society of Transplant Physicians (ASTP); 1997 May 10‐14; Chicago (ILL). 1997:261. [CENTRAL: CN‐00509206]
-
- Gaber LW, Moore LW, Gaber AO, Tesi RJ, Meyer J, Schroeder TJ. Correlation of histology to clinical rejection reversal: a thymoglobulin multicenter trial report. Kidney International 1999;55(6):2415‐22. [MEDLINE: ] - PubMed
-
- Gaber LW, US Multicenter Thymoglobulin Study Group. Correlation of post treatment renal allograft biopsies to rejection reversal [abstract]. 16th Annual Meeting. American Society of Transplant Physicians (ASTP); 1997 May 10‐14; Chicago (ILL). 1997:238. [CENTRAL: CN‐00509207]
Glass 1983 {published data only}
-
- Glass NR, Miller DT, Sollinger HW, Belzer FO. A comparative study of steroids and heterologous antiserum in the treatment of renal allograft rejection. Transplantation Proceedings 1983;15(1):617‐21. [EMBASE: 13086383]
Goldstein 1985 {published data only}
-
- A randomized clinical trial of OKT3 monoclonal antibody for acute rejection of cadaveric renal transplants. Ortho Multicenter Transplant Study Group. New England Journal of Medicine 1985;313(6):337‐42. [MEDLINE: ] - PubMed
-
- Cosimi AB. OKT3: First‐dose safety and success. Nephron 1987;46 Suppl 1:12‐8. [MEDLINE: ] - PubMed
-
- Goldstein G, Norman DJ, Shield CF 3rd, Kreis H, Burdick J, Flye MW, et al. OKT3 monoclonal antibody reversal of acute renal allograft rejection unresponsive to conventional immunosuppressive treatments. Progress in Clinical & Biological Research 1986;224:239‐49. [MEDLINE: ] - PubMed
Hesse 1990 {published data only}
-
- Hesse UJ, Wienand P, Baldamus C, Arns W. Preliminary results of a prospectively randomized trial of ALG vs OKT3 for steroid‐resistant rejection after renal transplantation in the early postoperative period. Transplantation Proceedings 1990;22(5):2273‐4. [MEDLINE: ] - PubMed
-
- Hesse UJ, Wienand P, Baldamus C, Pollok M, Pichlmaier H. The risk of infection following OKT3 and antilymphocyte globulin treatment for renal transplant rejection: results of a single center prospectively randomized trial. Transplant International 1992;5 Suppl 1:S440‐3. [MEDLINE: ] - PubMed
-
- Stippel DL, Arns W, Pollok M, Beckurts KT, Hesse UJ, Holscher AH. ALG versus OKT3 for treatment of steroid‐resistant rejection in renal transplantation: ten‐year follow‐up results of a randomized trial. Transplantation Proceedings 2002;34(6):2201‐2. [MEDLINE: ] - PubMed
Hilbrands 1996 {published data only}
-
- Hilbrands LB, Hoitsma AJ, Koene RA. Methylprednisolone versus ATG as initial treatment for acute rejections after renal transplantation [abstract]. Nephrology Dialysis Transplantation 1996;11(8):1675. [CENTRAL: CN‐00445721]
Hoitsma 1982 {published data only}
-
- Hoitsma AJ, Reekers P, Kreeftenberg JG, Lier HJ, Capel PJ, Koene RA. Treatment of acute rejection of cadaveric renal allografts with rabbit antithymocyte globulin. Transplantation 1982;33(1):12‐6. [MEDLINE: ] - PubMed
-
- Hoitsma AJ, Lier HJ, Reekers P, Koene RA. Improved patient and graft survival after treatment of acute rejections of cadaveric renal allografts with rabbit antithymocyte globulin. Transplantation 1985;39(3):274‐9. [MEDLINE: ] - PubMed
Hourmant 1985 {published data only}
-
- Hourmant M, Soulillou JP, Remi JP, Sagniez G, Guenel J. Use of cyclosporin A after antilymphocyte serum in renal transplantation [Utilisation de la cyclosporine A en relais du serum antilymphocytaire en transplantation renale]. Presse Medicale 1985;14(41):2093‐6. [MEDLINE: ] - PubMed
-
- Schneider T, Fagnani F, Lanoe JL, Hourmant M, Soulillou JP. Economic analysis of an immunosuppressive strategy in renal transplantation. Health Policy 1988;9(1):75‐89. [MEDLINE: ] - PubMed
Howard 1977 {published and unpublished data}
-
- Howard RJ, Condie RM, Sutherland DE, Simmons RL, Najarian JS. The use of antilymphoblast globulin in the treatment of renal allograft rejection. Transplantation Proceedings 1981;13(1 Pt 1):473‐4. [MEDLINE: ] - PubMed
-
- Howard RJ, Condie RM, Sutherland DE, Simmons RL, Najarian JS. The use of antilymphoblast globulin in the treatment of renal allograft rejection: a double‐blind, randomized study. Transplantation 1977;24(6):419‐23. [MEDLINE: ] - PubMed
Johnson 1989 {published data only}
-
- Johnson K, Niblack G, Richie R, MacDonell R, Nylander W, Walker P, et al. Multicenter comparison of rejection reversal: rabbit anti‐human lymphocyte serum (ATS) versus horse anti‐human lymphocyte globulin (ATGAM). Transplantation Proceedings 1989;21(1 Pt 2):1734‐5. [MEDLINE: ] - PubMed
-
- Johnson K, Niblack G, Vaughn W. The effectiveness of rabbit anti‐thymocyte serum (ATS) in the reversal of acute allograft rejection ‐ a multicenter study [abstract]. Kidney International 1987;31(1):460. [CENTRAL: CN‐00583631]
Mariat 1998 {published data only}
-
- Mariat C, Alamartine E, Diab N, Filippis JP, Laurent B, Berthoux F. A randomized prospective study comparing low‐dose OKT3 to low‐dose ATG for the treatment of acute steroid‐resistant rejection episodes in kidney transplant recipients. Transplant International 1998;11(3):231‐6. [MEDLINE: ] - PubMed
-
- Mariat C, Alamartine E, Laurent‐Pilonchery B, Diab N, Filippis JP, Berthoux F. Randomized prospective study comparing low‐dose OKT3 to low‐dose antithymocyte globulins for treatment of the first acute rejection of kidney allografts [abstract]. Nephrology Dialysis Transplantation 1996;11(6):276. [CENTRAL: CN‐00261331]
Midtvedt 1996 {published and unpublished data}
-
- Midtvedt K, Tafjord AB, Hartmann A, Eide TC, Holdaas H, Nordal KP, et al. Half dose of OKT3 is efficient in treatment of steroid‐resistant renal allograft rejection. Transplantation 1996;62(1):38‐42. [MEDLINE: ] - PubMed
-
- Midtvedt K, Tafjord AB, Nordal KP, Draganov B, Eide T, Hartmann A, et al. OKT3, doses of 2.5mg versus 5mg in steroid resistant renal allograft rejections [abstract]. Journal of the American Society of Nephrology 1995;6(3):1106. [CENTRAL: CN‐00485099]
Midtvedt 2003 {published and unpublished data}
-
- Fauchald P, Midtvedt K, Lien B, Hartmann A, Albrechtsen D, Bjerkely BL, et al. Randomized trial of T‐cell monitored administration of ATG vs OKT3 in steroid resistant kidney graft rejection [abstract]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00415630]
-
- Midtvedt K, Fauchald P, Lien B, Hartmann A, Albrechtsen D, Bjerkely BL, et al. Individualized T cell monitored administration of ATG versus OKT3 in steroid‐resistant kidney graft rejection. Clinical Transplantation 2003;17(1):69‐74. [MEDLINE: ] - PubMed
Okubo 1993 {published data only}
-
- Okubo M, Tamura K, Kamata K, Tsukamoto Y, Nakayama Y, Osakabe T, et al. 15‐Deoxyspergualin "rescue therapy" for methylprednisolone‐resistant rejection of renal transplants as compared with anti‐T cell monoclonal antibody (OKT3). Transplantation 1993;55(3):505‐8. [MEDLINE: ] - PubMed
Olausson 1995 {published data only}
-
- Olausson M, Mjornstedt L, Blohme I. Three‐day or ten‐day ATG treatment for steroid‐resistant rejection in kidney transplanted patients. Transplantation Proceedings 1995;27(6):3434‐5. [MEDLINE: ] - PubMed
-
- Olausson M, Mjornstedt L, Brynger H, Blohme I. Steroid‐resistant rejection in kidney‐transplanted patients: is ATG treatment for three or ten days preferable?. Transplant International 1996;9 Suppl 1:S38‐40. [MEDLINE: ] - PubMed
RITUX‐ERAH 2016 {published data only}
-
- Sautenet B, Blancho G, Buchler M, Morelon E, Toupance O, Barrou B, et al. One year results of the effects of rituximab on acute antibody‐mediated rejection in renal transplantation: RITUX ERAH, a multicenter double‐blind randomized placebo‐controlled trial. Transplantation 2016;100(2):391‐9. [MEDLINE: ] - PubMed
-
- Sautenet B, Blancho G, Buchler M, Morelon E, Toupance O, Barrou B, et al. One year results of the effects of rituximab on acute humoral rejection in renal transplantation: RITUX ERAH, a multicenter randomized placebo controlled trial [abstract no: 266]. American Journal of Transplantation 2013;13(Suppl S5):112. [EMBASE: 71056842] - PubMed
-
- Sautenet B, Blancho G, Buchler M, Morelon E, Toupance O, Barrou B, et al. RITUX‐ERAH: Multicenter randomized trial of rituximab on acute humoral rejection in transplantation [abstract no:O218]. Transplant International 2013;26(Suppl 2):110. [EMBASE: 71359364]
-
- Sautenet B, Blancho G, Buchler M, Morelon E, Toupance O, Barrou B, et al. RITUX‐ERAH: multicenter randomized trial of rituximab on acute antibody mediated rejection in transplantation [abstract no: O90]. Transplant International 2013;26(Suppl 3):22. [EMBASE: 71356165]
Shield 1979 {published data only}
-
- Shield CF 3rd, Cosimi AB, Tolkoff‐Rubin N, Rubin RH, Herrin J, Russell PS. Use of antithymocyte globulin for reversal of acute allograft rejection. Transplantation 1979;28(6):461‐4. [MEDLINE: ] - PubMed
Simonian 1983 {published data only}
-
- Simonian S, Lyons P, Chvala R, Swartz C, Onesti G, Bulova S. Reversal of acute cadaveric renal allograft rejection with adjunctive ATG treatment [abstract]. Kidney International 1983;23(1):295. [CENTRAL: CN‐00716036]
Spieker 1992 {published data only}
-
- Barenbrock M, Spieker C, Buchholz B, Heidenreich S, Zidek W, Rahn KH. Cardiovascular effects of the rejection therapy with antibodies against lymphocytes [Kardiovaskulare nebenwirkungen der abstossungsbehandlung mit lymphozytenantikorpern]. Nieren‐und Hochdruckkrankheiten 1994;23(2):84‐7. [EMBASE: 24091066]
-
- Spieker C, Barenbrock M, Buchholz B, Heidenreich S, Zidek W. Cardiovascular effects of ATG and OKT3 in renal allograft recipients. Transplantation Proceedings 1992;24(6):2594‐5. [MEDLINE: ] - PubMed
Streem 1983 {published data only}
-
- Streem SB, Novick AC, Braun WE, Steinmuller D, Greenstreet R. Low‐dose maintenance prednisone and antilymphoblast globulin for the treatment of acute rejection. A steroid‐sparing approach to immunosuppressive therapy. Transplantation 1983;35(5):420‐4. [MEDLINE: ] - PubMed
Theodorakis 1998 {published data only}
-
- Theodorakis J, Schneeberger H, Illner WD, Stangl M, Zanker B, Land W. Aggressive treatment of the first acute rejection episode using first‐line anti‐lymphocytic preparation reduces further acute rejection episodes after human kidney transplantation. Transplant International 1998;11 Suppl 1:S86‐9. [MEDLINE: ] - PubMed
Toledo‐Pereyra 1985 {published data only}
-
- Toledo‐Pereyra LH, Bergren C, Mittal VK, Whitten JI, Baskin S, McNichol L. A prospective randomized comparison of antilymphoblast globulin versus antithymocyte globulin for cadaver kidney transplantation. Transplantation 1985;40(4):448‐50. [MEDLINE: ] - PubMed
-
- Toledo‐Pereyra LH, Bergren C, Whitten J. Comparison of ALG and ATG for renal transplantation [abstract]. Kidney International 1985;28(2):388. [CENTRAL: CN‐00583280]
Waid 1991 {published data only}
-
- Lucas BA, Waid TH, Thompson JS, Brown SA, Munch LC, McKeown JW, et al. Comparison of T10Bg.1A‐31 and OKT3 in treating acute renal allograft rejection. Transplantation Proceedings 1993;25(1 Pt 1):543‐5. [MEDLINE: ] - PubMed
-
- Waid TH, Lucas BA, Thompson JS, Brown S, Munch LC, Kryscio R, et al. Treatment of acute rejection in renal allografts with t10b9.1a‐31 or OKT3 monoclonal antibody [abstract]. Journal of the American Society of Nephrology 1992;3(3):886. [CENTRAL: CN‐00461957]
-
- Waid TH, Lucas BA, Thompson JS, Brown SA, Munch L, Prebeck RJ, et al. Treatment of acute cellular rejection with T10B9.1A‐31 or OKT3 in renal allograft recipients. Transplantation 1992;53(1):80‐6. [MEDLINE: ] - PubMed
-
- Waid TH, Lucas BA, Thompson JS, McKeown JW, Brown S, Kryscio R, et al. Treatment of renal allograft rejection with T10B9.1A31 or OKT3: final analysis of a phase II clinical trial. Transplantation 1997;64(2):274‐81. [MEDLINE: ] - PubMed
-
- Waid TH, Lucas BA, Thompson JS, McKeown JW, Brown SA. Improved graft and patient survival with t10b9 1‐a or OKT3 monoclonal antibody for treatment for primary rejection [abstract]. Journal of the American Society of Nephrology 1994;5(3):1042. [CENTRAL: CN‐00679081]
Zarkhin 2008 {published data only}
-
- Sarwal M, Zarkhin V, Mohile S, Kambham N, Li L, Martin J, et al. Randomized trial of Rituximab vs standard of care for B cell dense acute renal transplant rejection [abstract no: 538]. American Journal of Transplantation 2007;7(Suppl 2):287. [CENTRAL: CN‐00644179]
-
- Zarkhin V, Li L, Kambham N, Sigdel T, Salvatierra O, Sarwal MM. A randomized, prospective trial of rituximab for acute rejection in pediatric renal transplantation. American Journal of Transplantation 2008;8(12):2607‐17. [MEDLINE: ] - PubMed
References to studies excluded from this review
Kulkarni 2016 {published data only}
-
- Kulkarni S, Kirkiles‐Smith NC, Deng YH, Formica RN, Moeckel G, Broecker V, et al. Eculizumab therapy for chronic antibody‐mediated injury in kidney transplant recipients: a pilot, randomized‐controlled trial. American Journal of Transplantation 2016;17(3):682‐91. [MEDLINE: ] - PubMed
References to ongoing studies
RIACT Study 2012 {published data only}
-
- Schiffer L, Schiffer M, Merkel S, Schwarz A, Mengel M, Jurgens C, et al. Rationale and design of the RIACT‐study: a multi‐center placebo controlled double blind study to test the efficacy of rItuximab in acute cellular tubulointerstitial rejection with B‐cell infiltrates in renal transplant patients: study protocol for a randomized controlled trial. Trials [Electronic Resource] 2012;13:199. [MEDLINE: ] - PMC - PubMed
Additional references
ANZDATA 2004
-
- Excell L, Chadban S, McDonald S. ANZDATA 27th Annual Report. Chapter 8: Transplantation. www.anzdata.org.au/anzdata/AnzdataReport/27thReport/files/Ch08Transplant... (accessed 9 May 2017).
ANZDATA 2012
-
- Clayton P, Campbell S, Chadban S, McDonald S, Hurst K. ANZDATA 35th Annual Report. Chapter 8: transplantation. www.anzdata.org.au/anzdata/AnzdataReport/35thReport/2012c08_transplants_... (accessed 9 May 2017).
Bartel 2011
-
- Bartel G, Schwaiger E, Bohmig GA. Prevention and treatment of alloantibody‐mediated kidney transplant rejection. Transplant International 2011;24(2):1142‐55. [MEDLINE: ] - PubMed
Basu 2005
-
- Basu A, Ramkumar M, Tan HP, Khan A, McCauley J, Marcos A, et al. Reversal of acute cellular rejection after renal transplantation with Campath‐1H. Transplantation Proceedings 2005;37(2):923‐6. [MEDLINE: ] - PubMed
Chan 2005
Chon 2014
-
- Chon WJ, Brennan DC. Acute renal allograft rejection: treatment. www.uptodate.com/contents/acute‐renal‐allograft‐rejection‐treatment (accessed 9 May 2017).
Colvin 2005
-
- Colvin RB, Smith RN. Antibody‐mediated organ‐allograft rejection. Nature Reviews. Immunology 2005;5(10):807‐17. [MEDLINE: ] - PubMed
Csapo 2005
-
- Csapo Z, Benavides‐Viveros C, Podder H, Pollard V, Kahan BD. Campath‐1H as rescue therapy for the treatment of acute rejection in kidney transplant patients. Transplantation Proceedings 2005;37(5):2032‐6. [MEDLINE: ] - PubMed
Cuervo 2003
Denton 1999
-
- Denton MD, Magee CC, Sayegh MH. Immunosuppressive strategies in transplantation. Lancet 1999;353(9158):1083‐91. [MEDLINE: ] - PubMed
Dheda 2013
-
- Dheda S, Chong S, Williams RL, Wong G, Lim WH. Chapter 5: Detection of antibody‐mediated rejection in kidney transplantation and the management of highly sensitised kidney transplant recipients. In: Rath T editor(s). Current Issues and Future Direction in Kidney Transplantation. InTech, 2013. [DOI: 10.5772/54735] - DOI
Egger 2001
-
- Egger M, Davey Smith G, Altman DG. Problems and limitations in conducting systematic reviews. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic Reviews in Health Care. 2nd Edition. London: BMJ Books, 2001:43‐68.
GRADE 2008
Haas 2016
-
- Haas M. The revised (2013) Banff classification for antibody‐mediated rejection of renal allografts: update, difficulties, and future considerations. American Journal of Transplantation 2016;16(5):1352‐7. [MEDLINE: ] - PubMed
Halloran 2004
-
- Halloran PF. Immunosuppressive drugs for kidney transplantation. [Review] [124 refs][Erratum appears in N Engl J Med. 2005 Mar 10;352(10):1056]. New England Journal of Medicine 2004;351(26):2715‐29. [MEDLINE: ] - PubMed
Hardinger 2013
Higgins 2003
Higgins 2011
-
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hollis 1999
Ingulli 2010
Issa 2010
-
- Issa F, Schiopu A, Wood KJ. Role of T cells in graft rejection and transplantation tolerance. Expert Review of Clinical Immunology 2010;6(1):155‐69. [MEDLINE: ] - PubMed
Jalalzadeh 2015
Jordan 2011
-
- Jordan SC, Toyoda M, Vo AA. Regulation of immunity and inflammation by intravenous immunoglobulin: relevance to solid organ transplantation. Expert Review of Clinical Immunology 2011;7(3):341‐8. [MEDLINE: ] - PubMed
Joseph 2001
-
- Joseph JT, Kingsmore DB, Junor BJ, Briggs JD, Mun Woo Y, Jaques BC, et al. The impact of late acute rejection after cadaveric kidney transplantation. Clinical Transplantation 2001;15(4):221‐7. [MEDLINE: ] - PubMed
Koo 2015
Loke 2001
Madden 2000
-
- Madden RL, Mulhern JG, Benedetto BJ, O'Shea MH, Germain MJ, Braden GL, et al. Completely reversed acute rejection is not a significant risk factor for the development of chronic rejection in renal allograft recipients. Transplant International 2000;13(5):344‐50. [MEDLINE: ] - PubMed
Masson 2014
Matas 2014
-
- Matas AJ, Smith JM, Skeans MA, Thompson B, Gustafson SK, Schnitzler MA, et al. OPTN/SRTR 2012 Annual Data Report: kidney. American Journal of Transplantation 2014;14 Suppl 1:11‐44. [MEDLINE: ] - PubMed
Mengel 2007
-
- Mengel M, Gwinner W, Schwarz A, Bajeski R, Franz I, Brocker V, et al. Infiltrates in protocol biopsies from renal allografts. American Journal of Transplantation 2007;7(2):356‐65. [MEDLINE: ] - PubMed
Nankivell 2010
-
- Nankivell BJ, Alexander SI. Rejection of the kidney allograft. New England Journal of Medicine 2010;363(15):1451‐62. [MEDLINE: ] - PubMed
NICE 2004
-
- Immunosuppressive therapy for renal transplantation in adults. London (UK): National Institute for Clinical Excellence (NICE); September 2004. Technology appraisal [TA85]. www.nice.org.uk/guidance/ta85 (accessed 9 May 2017).
Opelz 1997
-
- Opelz G. Critical evaluation of the association of acute with chronic graft rejection in kidney and heart transplant recipients. The Collaborative Transplant Study. Transplantation Proceedings 1997;29(1‐2):73‐6. [MEDLINE: ] - PubMed
Opelz 2008
-
- Opelz G, Dohler B, Collaborative Transplant Study Report. Influence of time of rejection on long‐term graft survival in renal transplantation. Transplantation 2008;85(5):661‐6. [MEDLINE: ] - PubMed
Peduzzi 1993
-
- Peduzzi P, Wittes J, Detre K, Holford T. Analysis as‐randomized and the problem of non‐adherence: an example from the Veterans Affairs Randomized Trial of Coronary Artery Bypass Surgery. Statistics in Medicine 1993;12(13):1185‐95. [MEDLINE: ] - PubMed
Roberts 2012
-
- Roberts DM, Jiang SH, Chadban SJ. The treatment of acute antibody‐mediated rejection in kidney transplant recipients‐a systematic review. Transplantation 2012;94(8):775‐83. [MEDLINE: ] - PubMed
Sackett 1979
-
- Sackett DL, Gent M. Controversy in counting and attributing events in clinical trials. New England Journal of Medicine 1979;301(26):1410‐2. [MEDLINE: ] - PubMed
Schünemann 2011a
-
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Schünemann 2011b
-
- Schünemann HJ, Oxman AD, Higgins JP, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Solez 1993
-
- Solez K, Axelsen RA, Benediktsson H, Burdick JF, Cohen AH, Colvin RB, et al. International standardization of criteria for the histologic diagnosis of renal allograft rejection: the Banff working classification of kidney transplant pathology. Kidney International 1993;44(2):411‐22. [MEDLINE: ] - PubMed
Solez 2008
-
- Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, et al. Banff 07 classification of renal allograft pathology: updates and future directions. American Journal of Transplantation 2008;8(4):753‐60. [MEDLINE: ] - PubMed
Szczech 1997
-
- Szczech LA, Berlin JA, Aradhye S, Grossman RA, Feldman HI. Effect of anti‐lymphocyte induction therapy on renal allograft survival: a meta‐analysis. Journal of the American Society of Nephrology 1997;8(11):1771‐7. [MEDLINE: ] - PubMed
Szczech 1998
-
- Szczech LA, Berlin JA, Feldman HI. The effect of antilymphocyte induction therapy on renal allograft survival. A meta‐analysis of individual patient‐level data. Anti‐Lymphocyte Antibody Induction Therapy Study Group. Annals of Internal Medicine 1998;128(10):817‐26. [MEDLINE: ] - PubMed
Tunis 2003
-
- Tunis SR, Stryer DB, Clancy CM. Practical clinical trials; increasing the value of clinical research for decision making in clinical and health policy. JAMA 2003;290(12):1624‐32. [MEDLINE: ] - PubMed
USRDS 2014
-
- National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD. 2014 USRDS Annual data report: Epidemiology of kidney disease in the United States Volume 2: End‐stage renal disease (ESRD) in the United States. Chapter 6: Transplantation. www.usrds.org/2014/view/v2_06.aspx (accessed 9 May 2017).
References to other published versions of this review
Webster 2004
Webster 2006a
Webster 2006b
-
- Webster AC, Pankhurst T, Rinaldi F, Chapman JR, Craig JC. Monoclonal and polyclonal antibody therapy for treating acute rejection in kidney transplant recipients: a systematic review of randomized trial data. Transplantation 2006;81(7):953‐65. [MEDLINE: ] - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

