Inversion recovery ultrashort echo time imaging of ultrashort T2 tissue components in ovine brain at 3 T: a sequential D2 O exchange study
- PMID: 28731616
- PMCID: PMC5617132
- DOI: 10.1002/nbm.3767
Inversion recovery ultrashort echo time imaging of ultrashort T2 tissue components in ovine brain at 3 T: a sequential D2 O exchange study
Abstract
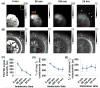
Inversion recovery ultrashort echo time (IR-UTE) imaging holds the potential to directly characterize MR signals from ultrashort T2 tissue components (STCs), such as collagen in cartilage and myelin in brain. The application of IR-UTE for myelin imaging has been challenging because of the high water content in brain and the possibility that the ultrashort T2 * signals are contaminated by water protons, including those associated with myelin sheaths. This study investigated such a possibility in an ovine brain D2 O exchange model and explored the potential of IR-UTE imaging for the quantification of ultrashort T2 * signals in both white and gray matter at 3 T. Six specimens were examined before and after sequential immersion in 99.9% D2 O. Long T2 MR signals were measured using a clinical proton density-weighted fast spin echo (PD-FSE) sequence. IR-UTE images were first acquired with different inversion times to determine the optimal inversion time to null the long T2 signals (TInull ). Then, at this TInull , images with echo times (TEs) of 0.01-4 ms were acquired to measure the T2 * values of STCs. The PD-FSE signal dropped to near zero after 24 h of immersion in D2 O. A wide range of TInull values were used at different time points (240-330 ms for white matter and 320-350 ms for gray matter at TR = 1000 ms) because the T1 values of the long T2 tissue components changed significantly. The T2 * values of STCs were 200-300 μs in both white and gray matter (comparable with the values obtained from myelin powder and its mixture with D2 O or H2 O), and showed minimal changes after sequential immersion. The ultrashort T2 * signals seen on IR-UTE images are unlikely to be from water protons as they are exchangeable with deuterons in D2 O. The source is more likely to be myelin itself in white matter, and might also be associated with other membranous structures in gray matter.
Keywords: T2*; gray matter; inversion recovery ultrashort echo time imaging; inversion time; myelin; white matter.
Copyright © 2017 John Wiley & Sons, Ltd.
Figures





References
-
- Koch-Henriksen N, Sorensen PS. The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurol. 2010;9(5):520–532. - PubMed
-
- Deoni SCL, Rutt BK, Arun T, Pierpaoli C, Jones DK. Gleaning Multicomponent T(1) and T(2) Information From Steady-State Imaging Data. Magn Reson Med. 2008;60(6):1372–1387. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

