The Colorful World of Extracellular Electron Shuttles
- PMID: 28731847
- PMCID: PMC5679407
- DOI: 10.1146/annurev-micro-090816-093913
The Colorful World of Extracellular Electron Shuttles
Abstract
Descriptions of the changeable, striking colors associated with secreted natural products date back well over a century. These molecules can serve as extracellular electron shuttles (EESs) that permit microbes to access substrates at a distance. In this review, we argue that the colorful world of EESs has been too long neglected. Rather than simply serving as a diagnostic attribute of a particular microbial strain, redox-active natural products likely play fundamental, underappreciated roles in the biology of their producers, particularly those that inhabit biofilms. Here, we describe the chemical diversity and potential distribution of EES producers and users, discuss the costs associated with their biosynthesis, and critically evaluate strategies for their economical usage. We hope this review will inspire efforts to identify and explore the importance of EES cycling by a wide range of microorganisms so that their contributions to shaping microbial communities can be better assessed and exploited.
Keywords: extracellular electron shuttle; metabolism; microbial diversity; public goods; redox chemistry.
Figures


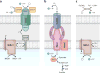

References
-
- Chemnetbase. Dictionary of Natural Products 25.2. Taylor and Francis; Oxford, UK: 2017. [accessed February, 2017]. http://dnp.chemnetbase.com/
-
- Alberty RA. Thermodynamics of the nitrogenase reactions. J. Biol. Chem. 1994;269:7099–102. - PubMed
-
- Amthor JS. The McCree–de Wit–Penning de Vries–Thornley respiration paradigms: 30 years later. Ann. Bot. 2000;86:1–20.
-
- Assary RS, Brushett FR, Curtiss LA. Reduction potential predictions of some aromatic nitrogen-containing molecules. RSC Adv. 2014;4:57442–51.
-
- Bacher A, Eberhardt S, Fischer M, Kis K, Richter G. Biosynthesis of vitamin B2 (riboflavin) Annu. Rev. Nutr. 2000;20:153–67. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

