Carboplatin with intravenous and subsequent oral administration of vinorelbine in resected non-small-cell-lung cancer in real-world set-up
- PMID: 28732018
- PMCID: PMC5521844
- DOI: 10.1371/journal.pone.0181803
Carboplatin with intravenous and subsequent oral administration of vinorelbine in resected non-small-cell-lung cancer in real-world set-up
Abstract
Objectives: Adjuvant cisplatin-based chemotherapy is recommended for routine use in patients with Stage IIA, IIB or IIIA non-small cell lung cancer (NSCLC) after complete resection. Results obtained for Stage IB were not conclusive. While vinorelbine plus cisplatin is the preferred choice after resection, combining vinorelbine with carboplatin promises improved compliance and delivery of drugs due to lower toxicity. We evaluated the impact of this option on treatment compliance and survival under real-world conditions.
Material and methods: A prospective, single-arm, multicenter, non-interventional study evaluated the tolerability, dose intensity and survival resulting from adjuvant use of intravenous carboplatin (AUC 5 on day 1) with vinorelbine administered both intravenously (25 mg/m2 on day 1) and orally (60 mg/m2 on day 8) within four cycles of 21 days each. A total of 74 patients with a median age of 64 years were observed.
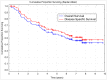
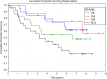
Results: The mean number of accomplished cycles was 3.78, and 62 patients (83.7%) completed all four planned cycles. Relative dose intensity for carboplatin was 88.9%, for intravenous vinorelbine 93.1%, and for oral vinorelbine 83.2%. Median follow-up was 4.73 years. Median disease-specific survival (DSS) was 7.63 years, median overall survival (OS) was 5.90 years, median disease-free survival (DFS0) was 4.43 years, and five-year survival was 56.2%. TNM stage of disease significantly affected DSS and OS. Favorable survival was observed in females, nonsmokers, patients aged over 65 years, patient with prior lobectomy, patients with tumor of squamous histology, and those who finished the planned therapy, but the differences were non-significant.
Conclusion: Adjuvant carboplatin with vinorelbine switched from intravenous to oral administration was shown to be a favorable regimen with regard to tolerability and safety. Compliance to therapy was high, and survival parameters were promising, showing that applied regimen can be another potential option for adjuvant chemotherapy in patients with NSCLC.
Conflict of interest statement
Figures
Similar articles
-
Intravenous or oral administration of vinorelbine in adjuvant chemotherapy with cisplatin and vinorelbine for resected NSCLC.Lung Cancer. 2015 May;88(2):167-73. doi: 10.1016/j.lungcan.2015.02.010. Epub 2015 Feb 21. Lung Cancer. 2015. PMID: 25769883
-
Phase II study of oral vinorelbine in combination with carboplatin followed by consolidation therapy with oral vinorelbine as single-agent in unresectable localized or metastatic non-small cell lung carcinoma.Lung Cancer. 2009 Jun;64(3):319-25. doi: 10.1016/j.lungcan.2008.10.014. Epub 2008 Dec 17. Lung Cancer. 2009. PMID: 19095327 Clinical Trial.
-
Adjuvant carboplatin-based chemotherapy in resected stage IIIA-N2 non-small cell lung cancer.J Thorac Oncol. 2010 Jul;5(7):1033-41. doi: 10.1097/JTO.0b013e3181d95db4. J Thorac Oncol. 2010. PMID: 20502361 Clinical Trial.
-
[Oral vinorelbine: pharmacology and treatment outcome in non-small cell bronchial carcinoma and breast carcinoma].Onkologie. 2006 Mar;29 Suppl 1:1-28. doi: 10.1159/000091889. Epub 2006 Mar 3. Onkologie. 2006. PMID: 16534241 Review. German.
-
Compliance with post-operative adjuvant chemotherapy in non-small cell lung cancer. An analysis of National Cancer Institute of Canada and intergroup trial JBR.10 and a review of the literature.Lung Cancer. 2005 Mar;47(3):385-94. doi: 10.1016/j.lungcan.2004.08.016. Lung Cancer. 2005. PMID: 15713522 Review.
Cited by
-
Analysis of the Management Effect of Cancer Patients after Oral Chemotherapy Based on Nursing Process Reengineering.J Healthc Eng. 2022 Mar 22;2022:4539125. doi: 10.1155/2022/4539125. eCollection 2022. J Healthc Eng. 2022. Retraction in: J Healthc Eng. 2023 Aug 16;2023:9764691. doi: 10.1155/2023/9764691. PMID: 35360484 Free PMC article. Retracted.
-
Oral Chemotherapy for Treatment of Lung Cancer.Front Oncol. 2020 Apr 28;10:793. doi: 10.3389/fonc.2020.00793. eCollection 2020. Front Oncol. 2020. PMID: 32426292 Free PMC article. Review.
-
Application of Approved Cisplatin Derivatives in Combination Therapy against Different Cancer Diseases.Molecules. 2022 Apr 11;27(8):2466. doi: 10.3390/molecules27082466. Molecules. 2022. PMID: 35458666 Free PMC article. Review.
References
-
- Coello MC, Luketich JD, Litle VR, Godfrey, Tony E. Prognostic significance of micrometastasis in non-small-cell lung cancer. Clin Lung Cancer. 2004; 5(4): 214–225. doi: 10.3816/CLC.2004.n.002 - DOI - PubMed
-
- Siegel R, Ward E, Brawley O, Jemal A. Cancer Statistics. 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011; 61(4): 212–236. doi: 10.3322/caac.20121 - DOI - PubMed
-
- Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007; 2(8): 706–714. doi: 10.1097/JTO.0b013e31812f3c1a - DOI - PubMed
-
- Scagliotti GV, Fossati R, Torri V, Crino L, Giaccone G, Silvano G, et al. Randomized study of adjuvant chemotherapy for completely resected stage I, II, or IIIA non-small cell lung cancer. J Natl Cancer Inst. 2003; 95(19): 1453–1461. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical