The exported chaperone Hsp70-x supports virulence functions for Plasmodium falciparum blood stage parasites
- PMID: 28732045
- PMCID: PMC5521827
- DOI: 10.1371/journal.pone.0181656
The exported chaperone Hsp70-x supports virulence functions for Plasmodium falciparum blood stage parasites
Abstract
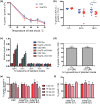
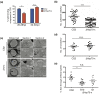
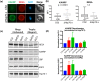
Malaria is caused by five different Plasmodium spp. in humans each of which modifies the host erythrocyte to survive and replicate. The two main causes of malaria, P. falciparum and P. vivax, differ in their ability to cause severe disease, mainly due to differences in the cytoadhesion of infected erythrocytes (IE) in the microvasculature. Cytoadhesion of P. falciparum in the brain leads to a large number of deaths each year and is a consequence of exported parasite proteins, some of which modify the erythrocyte cytoskeleton while others such as PfEMP1 project onto the erythrocyte surface where they bind to endothelial cells. Here we investigate the effects of knocking out an exported Hsp70-type chaperone termed Hsp70-x that is present in P. falciparum but not P. vivax. Although the growth of Δhsp70-x parasites was unaffected, the export of PfEMP1 cytoadherence proteins was delayed and Δhsp70-x IE had reduced adhesion. The Δhsp70-x IE were also more rigid than wild-type controls indicating changes in the way the parasites modified their host erythrocyte. To investigate the cause of this, transcriptional and translational changes in exported and chaperone proteins were monitored and some changes were observed. We propose that PfHsp70-x is not essential for survival in vitro, but may be required for the efficient export and functioning of some P. falciparum exported proteins.
Conflict of interest statement
Figures



References
-
- World Malaria Report [Internet]. 2016.
-
- Newbold C, Craig A, Kyes S, Rowe A, Fernandez-Reyes D, Fagan T. Cytoadherence, pathogenesis and the infected red cell surface in Plasmodium falciparum. Int J Parasitol. 1999;29(6):927–37. . - PubMed
-
- Rogerson SJ, Hviid L, Duffy PE, Leke RF, Taylor DW. Malaria in pregnancy: pathogenesis and immunity. Lancet Infect Dis. 2007;7(2):105–17. doi: 10.1016/S1473-3099(07)70022-1 . - DOI - PubMed
-
- Umbers AJ, Aitken EH, Rogerson SJ. Malaria in pregnancy: small babies, big problem. Trends Parasitol. 2011;27(4):168–75. doi: 10.1016/j.pt.2011.01.007 . - DOI - PubMed
-
- Maier AG, Rug M, O'Neill MT, Brown M, Chakravorty S, Szestak T, et al. Exported Proteins Required for Virulence and Rigidity of Plasmodium falciparum-Infected Human Erythrocytes. Cell. 2008;134(1):48–61. doi: 10.1016/j.cell.2008.04.051 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

