Efficacy of oral pre-exposure prophylaxis (PrEP) for HIV among women with abnormal vaginal microbiota: a post-hoc analysis of the randomised, placebo-controlled Partners PrEP Study
- PMID: 28732773
- PMCID: PMC5649365
- DOI: 10.1016/S2352-3018(17)30110-8
Efficacy of oral pre-exposure prophylaxis (PrEP) for HIV among women with abnormal vaginal microbiota: a post-hoc analysis of the randomised, placebo-controlled Partners PrEP Study
Abstract
Background: Daily oral tenofovir-based pre-exposure prophylaxis (PrEP) is high efficacious for HIV prevention among women with high adherence. However, the effect of abnormal vaginal microbiota on PrEP efficacy is of concern. We investigated whether bacterial vaginosis modified the efficacy of oral PrEP.
Methods: We used prospectively collected data from women in the Partners PrEP Study, a placebo-controlled trial of daily oral PrEP (either tenofovir monotherapy or a combination of tenofovir and emtricitabine) in HIV serodiscordant couples aged 18 years or older in Kenya and Uganda that showed high efficacy in women. We used Cox proportional hazards regression to assess PrEP efficacy among subgroups of women defined by bacterial vaginosis status based on yearly microscopy and Nugent scoring (0-3 indicated healthy microbiota, 4-6 intermediate, and 7-10 bacterial vaginosis). In separate efficacy analyses, we also investigated individual components of the score (ie, detection of Gardnerella vaginalis or Bacteroides spp and non-detection of Lactobacillus spp) as markers of abnormal microbiota.
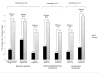
Findings: Of 1470 women (median age 33 years), 357 (24%) had bacterial vaginosis at enrolment. 45 women seroconverted to HIV. The HIV prevention efficacy of PrEP did not differ significantly among women with healthy microbiota (incidence 0·6 per 100 person years in PrEP group and 2·5 per 100 person-years in the placebo group; efficacy 76·55% [95% CI 43·09 to 90·37]), intermediate microbiota (HIV incidence 1·8 per 100 person-years in the PrEP group and 3·5 per 100 person-years in the placebo group; efficacy 62·72% [95% CI -66·59 to 91·66]), or bacterial vaginosis (HIV incidence 0·9 per 100 person-years in the PrEP group and 3·5 per 100 person-years in the placebo group; efficacy 72·50% [95% CI 5·98 to 91·95]; pinteraction=0·871). PrEP efficacy was not significantly different between women with detected G vaginalis or Bacteroides spp morphotypes and those without these morphotypes (efficacy 68·62% vs 76·72%; pinteraction=0·652); or between those with Lactobacillus spp morphotypes and those without (70·48% vs 74·08%; pinteraction=0·86).
Interpretation: Among African women with a high prevalence of bacterial vaginosis and high adherence to PrEP, the efficacy of daily oral PrEP for HIV prevention did not differ significantly among women with abnormal versus healthy vaginal microbiota as defined by Nugent score. These data are reassuring that oral PrEP delivery to women can continue without the need for concurrent testing for bacterial vaginosis or vaginal dysbiosis.
Funding: Bill & Melinda Gates Foundation, Eunice Kennedy Shriver National Institute of Child Health and Human Development, and National Institute of Allergy and Infectious Diseases.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Conflict of interest statement
R.S.M. has received research funding from Hologic Corporation, paid as a grant to the University of Washington. All other authors declare no conflicts of interest.
Figures


Comment in
-
Vaginal dysbiosis and pre-exposure prophylaxis efficacy.Lancet HIV. 2017 Oct;4(10):e427-e429. doi: 10.1016/S2352-3018(17)30130-3. Epub 2017 Jul 18. Lancet HIV. 2017. PMID: 28732774 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

