Injectable naltrexone, oral naltrexone, and buprenorphine utilization and discontinuation among individuals treated for opioid use disorder in a United States commercially insured population
- PMID: 28733097
- PMCID: PMC5750108
- DOI: 10.1016/j.jsat.2017.07.001
Injectable naltrexone, oral naltrexone, and buprenorphine utilization and discontinuation among individuals treated for opioid use disorder in a United States commercially insured population
Abstract
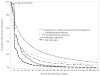
We investigated prescribing patterns for four opioid use disorder (OUD) medications: 1) injectable naltrexone, 2) oral naltrexone, 3) sublingual or oralmucosal buprenorphine/naloxone, and 4) sublingual buprenorphine as well as transdermal buprenorphine (which is approved for treating pain, but not OUD) in a nationally representative claims-based database (Truven Health MarketScan®) of commercially insured individuals in the United States. We calculated the prevalence of OUD in the database for each year from 2010 to 2014 and the proportion of diagnosed patient months on OUD medication. We compared characteristics of individuals diagnosed with OUD who did and did not receive these medications with bivariate descriptive statistics. Finally, we fit a Cox proportional hazards model of time to discontinuation of therapy as a function of therapy type, controlling for relevant confounders. From 2010 to 2014, the proportion of commercially insured individuals diagnosed with OUD grew by fourfold (0.12% to 0.48%), but the proportion of diagnosed patient-months on medication decreased from 25% in 2010 (0.05% injectable naltrexone, 0.4% oral naltrexone, 23.1% sublingual or oralmucosal buprenorphine/naloxone, 1.5% sublingual buprenorphine, and 0% transdermal buprenorphine) to 16% in 2014 (0.2% injectable naltrexone, 0.4% oral naltrexone, 13.8% sublingual or oralmucosal buprenorphine/naloxone, 1.4% sublingual buprenorphine, and 0.3% transdermal buprenorphine). Individuals who received medication therapy were more likely to be male, younger, and have an additional substance use disorder compared with those diagnosed with OUD who did not receive medication therapy. Those prescribed injectable naltrexone were more often male, younger, and diagnosed with additional substance use disorders compared with those prescribed other medications for opioid use disorder (MOUDs). At 30 days after initiation, 52% for individuals treated with injectable naltrexone, 70% for individuals treated with oral naltrexone, 31% for individuals treated with sublingual or oralmucosal buprenorphine/naloxone, 58% for individuals treated with sublingual buprenorphine, and 51% for individuals treated with transdermal buprenorphine discontinued treatment. In the Cox proportional hazard model, use of injectable naltrexone, oral naltrexone, sublingual buprenorphine, and transdermal buprenorphine were all associated with significantly greater hazard of discontinuing therapy beginning >30days after MOUD initiation (HR=2.17, 2.54, 1.15, and 2.21, respectively, 95% CIs 2.04-2.30, 2.45-2.64, 1.10-1.19, and 2.11-2.33), compared with the use of sublingual or oralmucosal buprenorphine/naloxone. This analysis demonstrates that the use of evidence-based medication therapies has not kept pace with increases in OUD diagnoses in commercially insured populations in the United States. Among those who have been treated, discontinuation rates >30days after initiation are high. The proportion treated with injectable naltrexone, oral naltrexone, and transdermal buprenorphine grew over time but remains small, and the discontinuation rates are higher among those treated with these medications compared with those treated with sublingual or oralmucosal buprenorphine/naloxone. In the face of the opioid overdose and addiction crisis, new efforts are needed at the provider, health system, and policy levels so that MOUD availability and uptake keep pace with new OUD diagnoses and OUD treatment discontinuation is minimized.
Keywords: buprenorphine; buprenorphine/naloxone; injectable naltrexone; opioid use disorder; oral naltrexone; treatment discontinuation.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures
References
-
- Allison P. Survival Analysis Using SAS: A Practical Guide. 2. Cary, NC, USA: SAS Institute Inc; 2010.
-
- Cochran BN, Flentje A, Heck NC, Van Den Bos J, Perlman D, Torres J, … Carter J. Factors predicting development of opioid use disorders among individuals who receive an initial opioid prescription: Mathematical modeling using a database of commercially-insured individuals. Drug Alcohol Depend. 2014;138:202–208. doi: 10.1016/j.drugalcdep.2014.02.701. - DOI - PMC - PubMed
-
- Cousins SJ, Radfar SR, Crevecoeur-MacPhail D, Ang A, Darfler K, Rawson RA. Predictors of Continued Use of Extended-Released Naltrexone (XR-NTX) for Opioid-Dependence: An Analysis of Heroin and Non-Heroin Opioid Users in Los Angeles County. J Subst Abuse Treat. 2016;63:66–71. doi: 10.1016/j.jsat.2015.12.004. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

