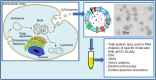
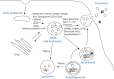
Current knowledge on exosome biogenesis and release
- PMID: 28733901
- PMCID: PMC5756260
- DOI: 10.1007/s00018-017-2595-9
Current knowledge on exosome biogenesis and release
Abstract
Exosomes are nanosized membrane vesicles released by fusion of an organelle of the endocytic pathway, the multivesicular body, with the plasma membrane. This process was discovered more than 30 years ago, and during these years, exosomes have gone from being considered as cellular waste disposal to mediate a novel mechanism of cell-to-cell communication. The exponential interest in exosomes experienced during recent years is due to their important roles in health and disease and to their potential clinical application in therapy and diagnosis. However, important aspects of the biology of exosomes remain unknown. To explore the use of exosomes in the clinic, it is essential that the basic molecular mechanisms behind the transport and function of these vesicles are better understood. We have here summarized what is presently known about how exosomes are formed and released by cells. Moreover, other cellular processes related to exosome biogenesis and release, such as autophagy and lysosomal exocytosis are presented. Finally, methodological aspects related to exosome release studies are discussed.
Keywords: Autophagy; Endosomes; Exosomes; Extracellular vesicles; Lysosomes; MVB biogenesis; MVBs; Microvesicles; Release; Secretion.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
-
- Yanez-Mo M, Siljander PR, Andreu Z, Zavec AB, Borras FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, Colas E, Cordeiro-da Silva A, Fais S, Falcon-Perez JM, Ghobrial IM, Giebel B, Gimona M, Graner M, Gursel I, Gursel M, Heegaard NH, Hendrix A, Kierulf P, Kokubun K, Kosanovic M, Kralj-Iglic V, Kramer-Albers EM, Laitinen S, Lasser C, Lener T, Ligeti E, Line A, Lipps G, Llorente A, Lotvall J, Mancek-Keber M, Marcilla A, Mittelbrunn M, Nazarenko I, Nolte-’t Hoen EN, Nyman TA, O’Driscoll L, Olivan M, Oliveira C, Pallinger E, Del Portillo HA, Reventos J, Rigau M, Rohde E, Sammar M, Sanchez-Madrid F, Santarem N, Schallmoser K, Ostenfeld MS, Stoorvogel W, Stukelj R, Van der Grein SG, Vasconcelos MH, Wauben MH, De Wever O. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066. doi: 10.3402/jev.v4.27066. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

