Amyloid β concentrations and stable isotope labeling kinetics of human plasma specific to central nervous system amyloidosis
- PMID: 28734653
- PMCID: PMC5567785
- DOI: 10.1016/j.jalz.2017.06.2266
Amyloid β concentrations and stable isotope labeling kinetics of human plasma specific to central nervous system amyloidosis
Erratum in
-
Corrigendum.Alzheimers Dement. 2017 Oct;13(10):1185. doi: 10.1016/j.jalz.2017.09.004. Alzheimers Dement. 2017. PMID: 29029683 No abstract available.
Abstract
Introduction: Cerebrospinal fluid analysis and other measurements of amyloidosis, such as amyloid-binding positron emission tomography studies, are limited by cost and availability. There is a need for a more practical amyloid β (Aβ) biomarker for central nervous system amyloid deposition.
Methods: We adapted our previously reported stable isotope labeling kinetics protocol to analyze the turnover kinetics and concentrations of Aβ38, Aβ40, and Aβ42 in human plasma.
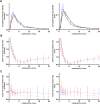
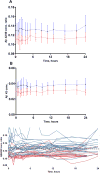
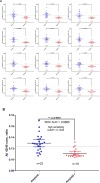
Results: Aβ isoforms have a half-life of approximately 3 hours in plasma. Aβ38 demonstrated faster turnover kinetics compared with Aβ40 and Aβ42. Faster fractional turnover of Aβ42 relative to Aβ40 and lower Aβ42 and Aβ42/Aβ40 concentrations in amyloid-positive participants were observed.
Discussion: Blood plasma Aβ42 shows similar amyloid-associated alterations as we have previously reported in cerebrospinal fluid, suggesting a blood-brain transportation mechanism of Aβ. The stability and sensitivity of plasma Aβ measurements suggest this may be a useful screening test for central nervous system amyloidosis.
Keywords: Amyloid β; Aβ42; Human; Kinetics; Plasma; Turnover.
Copyright © 2017 the Alzheimer's Association. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Potter R, Patterson BW, Elbert DL, Ovod V, Kasten T, Sigurdson W, Mawuenyega K, Blazey T, Goate A, Chott R, Yarasheski KE, Holtzman DM, Morris JC, Benzinger TL, Bateman RJ. Increased in vivo amyloid-β42 production, exchange, and loss in presenilin mutation carriers. Sci Transl Med. 2013;5(189) - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

