P/Q-type voltage-gated calcium channels mediate the ethanol and CRF sensitivity of central amygdala GABAergic synapses
- PMID: 28734867
- PMCID: PMC5837809
- DOI: 10.1016/j.neuropharm.2017.07.017
P/Q-type voltage-gated calcium channels mediate the ethanol and CRF sensitivity of central amygdala GABAergic synapses
Abstract
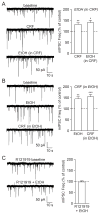
The central amygdala (CeA) GABAergic system is hypothesized to drive the development of alcohol dependence, due to its pivotal roles in the reinforcing actions of alcohol and the expression of negative emotion, anxiety and stress. Recent work has also identified an important role for the CeA corticotropin-releasing factor (CRF) system in the interaction between anxiety/stress and alcohol dependence. We have previously shown that acute alcohol and CRF each increase action potential-independent GABA release in the CeA via their actions at presynaptic CRF type 1 receptors (CRF1s); however, the shared mechanism employed by these two compounds requires further investigation. Here we report that acute alcohol interacts with the CRF/CRF1 system, such that CRF and alcohol act via presynaptic CRF1s and P/Q-type voltage-gated calcium channels to promote vesicular GABA release and that both compounds occlude the effects of each other at these synapses. Chronic alcohol exposure does not alter P/Q-type voltage-gated calcium channel membrane abundance or this CRF1/P/Q-type voltage-gated calcium channel mechanism of acute alcohol-induced GABA release, indicating that alcohol engages this molecular mechanism at CeA GABAergic synapses throughout the transition to dependence. Thus, P/Q-type voltage-gated calcium channels, like CRF1s, are key regulators of the effects of alcohol on GABAergic signaling in the CeA.
Keywords: Alcohol dependence; Alcohol/ethanol; Central amygdala; Corticotropin-releasing factor (CRF); Corticotropin-releasing factor type 1 receptor (CRF(1)); GABA; P/Q-type voltage-gated calcium channel.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures



References
-
- Alheid GF. Extended amygdala and basal forebrain. Ann N Y Acad Sci. 2003;985:185–205. - PubMed
-
- Belia S, Mannucci R, Lisciarelli M, Cacchio M, Fano G. Double effect of ethanol on intracellular Ca2+ levels in undifferentiated PC12 cells. Cell Signal. 1995;7:389–395. - PubMed
-
- Blank T, Nijholt I, Grammatopoulos DK, Randeva HS, Hillhouse EW, Spiess J. Corticotropin-releasing factor receptors couple to multiple G-proteins to activate diverse intracellular signaling pathways in mouse hippocampus: role in neuronal excitability and associative learning. J Neurosci. 2003;23:700–707. - PMC - PubMed
-
- Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–555. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

