Promoting and Protecting Public Health: How the European Union Pharmacovigilance System Works
- PMID: 28735357
- PMCID: PMC5606958
- DOI: 10.1007/s40264-017-0572-8
Promoting and Protecting Public Health: How the European Union Pharmacovigilance System Works
Abstract
This article provides an overview of the European Union pharmacovigilance system resulting from the rationalisation and strengthening delivered through the implementation of the revised pharmacovigilance legislation. It outlines the system aims, underlying principles, components and drivers for future change. At its core, the Pharmacovigilance Risk Assessment Committee is responsible for assessing all aspects of the risk management of medicinal products, thus ensuring that medicines approved for the European Union market are optimally used by maximising their benefits and minimising risks. The main objectives of the system are to promote and protect public health by supporting the availability of medicines including those that fulfil previously unmet medical needs, and reducing the burden of adverse drug reactions. These are achieved through a proactive, risk proportionate and patient-centred approach, with high levels of transparency and engagement of civil society. In the European Union, pharmacovigilance is now fully integrated into the life cycle of medicinal products, with the planning of pharmacovigilance activities commencing before a medicine is placed on the market, and companies encouraged to start planning very early in development for high-innovation products. After authorisation, information on the safety of medicines continues to be obtained through a variety of sources, including spontaneous reports of adverse drug reactions or monitoring real-world data. Finally, the measurement of the impact of pharmacovigilance activities, auditing and inspections, as well as capacity building ensure that the system undergoes continuous improvement and can always rely on the best methodologies to safeguard public health.
Conflict of interest statement
Funding
No sources of funding were used to assist in the preparation of this review.
Conflict of interest
Aniello Santoro, Georgy Genov and Peter Arlett are employees of the European Medicines Agency. Almath Spooner and June M. Raine are members of the Pharmacovigilance Risk Assessment Committee, as well as employees of the Health Products Regulatory Authority and the Medicines and Healthcare Products Regulatory Agency, respectively.
Disclaimer
The views expressed in this article are the personal views of the authors and may not be understood or quoted as being made on behalf of or reflecting the position of the agencies or organizations with which the authors are affiliated.
Figures



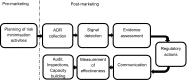


References
-
- World Health Organization. Pharmacovigilance. http://www.who.int/medicines/areas/quality_safety/safety_efficacy/pharmv... (2017). Accessed 3 Jul 2017.
-
- European Commission. MEMO/08/782, Brussels, 10 December November 2008. Strengthening pharmacovigilance to reduce adverse effects of medicines. http://europa.eu/rapid/press-release_MEMO-08-782_en.htm?locale=en (2017). Accessed 3 Jul 2017.
-
- European Commission. Pharmacovigilance. http://ec.europa.eu/health/human-use/pharmacovigilance/index_en.htm (2017). Accessed 3 Jul 2017.
-
- European Medicines Agency. Pharmacovigilance Risk Assessment Committee (PRAC). http://www.ema.europa.eu/ema/index.jsp?curl=pages/about_us/general/gener... (2017). Accessed 3 Jul 2017.
-
- European Medicines Agency. Good pharmacovigilance practices. http://www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/document_li... (2017). Accessed 3 Jul 2017.
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

