Transition-Metal-Free Decarboxylative Iodination: New Routes for Decarboxylative Oxidative Cross-Couplings
- PMID: 28735532
- PMCID: PMC5662929
- DOI: 10.1021/jacs.7b05155
Transition-Metal-Free Decarboxylative Iodination: New Routes for Decarboxylative Oxidative Cross-Couplings
Abstract
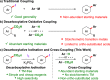
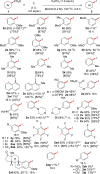
Constructing products of high synthetic value from inexpensive and abundant starting materials is of great importance. Aryl iodides are essential building blocks for the synthesis of functional molecules, and efficient methods for their synthesis from chemical feedstocks are highly sought after. Here we report a low-cost decarboxylative iodination that occurs simply from readily available benzoic acids and I2. The reaction is scalable and the scope and robustness of the reaction is thoroughly examined. Mechanistic studies suggest that this reaction does not proceed via a radical mechanism, which is in contrast to classical Hunsdiecker-type decarboxylative halogenations. In addition, DFT studies allow comparisons to be made between our procedure and current transition-metal-catalyzed decarboxylations. The utility of this procedure is demonstrated in its application to oxidative cross-couplings of aromatics via decarboxylative/C-H or double decarboxylative activations that use I2 as the terminal oxidant. This strategy allows the preparation of biaryls previously inaccessible via decarboxylative methods and holds other advantages over existing decarboxylative oxidative couplings, as stoichiometric transition metals are avoided.
Conflict of interest statement
The authors declare no competing financial interest.
Figures









References
-
- Hassan J.; Sévignon M.; Gozzi C.; Schulz E.; Lemaire M. Chem. Rev. 2002, 102, 1359–1470. 10.1021/cr000664r. - DOI - PubMed
- Johansson Seechurn C. C. C.; Kitching M. O.; Colacot T. J.; Snieckus V. Angew. Chem., Int. Ed. 2012, 51, 5062–5085. 10.1002/anie.201107017. - DOI - PubMed
- Bolm C.; Hildebrand J. P.; Muniz K.; Hermanns N. Angew. Chem., Int. Ed. 2001, 40, 3284–3308. 10.1002/1521-3773(20010917)40:18<3284::AID-ANIE3284>3.0.CO;2-U. - DOI - PubMed
-
- Bringmann G.; Günther C.; Ochse M.; Schupp O.; Tasler S.. Biaryls in Nature: A Multi-Facetted Class of Stereochemically, Biosynthetically, and Pharmacologically Intriguing Secondary Metabolites. In Progress in the Chemistry of Organic Natural Products; Herz W., Falk H., Kirby G. W., Moore R. E., Eds.; Springer: Vienna, 2001; Vol. 82, pp 1–249. - PubMed
- Horton D. A.; Bourne G. T.; Smythe M. L. Chem. Rev. 2003, 103, 893–930. 10.1021/cr020033s. - DOI - PubMed
- Grimsdale A. C.; Chan K. L.; Martin R. E.; Jokisz P. G.; Holmes A. B. Chem. Rev. 2009, 109, 897–1091. 10.1021/cr000013v. - DOI - PubMed
-
-
For selected reviews on C–H activation see:
- Alberico D.; Scott M. E.; Lautens M. Chem. Rev. 2007, 107, 174–238. 10.1021/cr0509760. - DOI - PubMed
- Ackermann L.; Vicente R.; Kapdi A. R. Angew. Chem., Int. Ed. 2009, 48, 9792–9826. 10.1002/anie.200902996. - DOI - PubMed
- Boorman T. C.; Larrosa I. Chem. Soc. Rev. 2011, 40, 1910–1925. 10.1039/C0CS00098A. - DOI - PubMed
- Wencel-Delord J.; Dröge T.; Liu F.; Glorius F. Chem. Soc. Rev. 2011, 40, 4740–4761. 10.1039/c1cs15083a. - DOI - PubMed
- Kuhl N.; Hopkinson M. N.; Wencel-Delord J.; Glorius F. Angew. Chem., Int. Ed. 2012, 51, 10236–10254. 10.1002/anie.201203269. - DOI - PubMed
- Kakiuchi F.; Kochi T.; Murai S. Synlett 2014, 25, 2390–2414. 10.1055/s-0034-1379210. - DOI
- Ahlsten N.; Cambeiro X. C.; Perry G. J. P.; Larrosa I. In Topics in Heterocyclic Chemistry; Bandini M., Ed.; Springer International Publishing: Berlin, 2016; Vol. 46, pp 175–226.
- Font M.; Quibell J. M.; Perry G. J. P.; Larrosa I. Chem. Commun. 2017, 53, 5584–5597. 10.1039/C7CC01755C. - DOI - PubMed
- Simonetti M.; Cannas D. M.; Larrosa I. In Advances in Organometallic Chemistry; Pérez P. J., Ed.; Elsevier: Amsterdam,2017; Vol. 67, pp 299–399.
- Murakami K.; Perry G. J. P.; Itami K. Org. Biomol. Chem. 2017, 15, 6071–6075. 10.1039/C7OB00985B. - DOI - PubMed
- Yi H.; Zhang G.; Wang H.; Huang Z.; Wang J.; Singh A. K.; Lei A. Chem. Rev. 2017, 117, 9016–9085. 10.1021/acs.chemrev.6b00620. - DOI - PubMed
-
-
-
For selected reviews on decarboxylative activation see:
- Gooßen L. J.; Gooßen K.; Rodríguez N.; Blanchot M.; Linder C.; Zimmermann B. Pure Appl. Chem. 2008, 80, 1725–1733. 10.1351/pac200880081725. - DOI
- Gooßen L. J.; Rodríguez N.; Gooßen K. Angew. Chem., Int. Ed. 2008, 47, 3100–3120. 10.1002/anie.200704782. - DOI - PubMed
- Gooßen L. J.; Collet F.; Gooßen K. Isr. J. Chem. 2010, 50, 617–629. 10.1002/ijch.201000039. - DOI
- Shang R.; Liu L. Sci. China: Chem. 2011, 54, 1670–1687. 10.1007/s11426-011-4381-0. - DOI
- Rodríguez N.; Gooßen L. J. Chem. Soc. Rev. 2011, 40, 5030–5048. 10.1039/c1cs15093f. - DOI - PubMed
- Dzik W. I.; Lange P. P.; Gooßen L. J. Chem. Sci. 2012, 3, 2671–2678. 10.1039/c2sc20312j. - DOI
- Cornella J.; Larrosa I. Synthesis 2012, 44, 653–676. 10.1055/s-0031-1289686. - DOI
- Gooßen L. J.; Gooßen K.. Decarboxylative Coupling Reactions. In Topics in Organometallic Chemistry; Gooßen L. J., Ed.; Springer-Verlag: Berlin/Heidelberg, 2013; Vol. 44, pp 121–142.
- Perry G. J. P.; Larrosa I. Eur. J. Org. Chem. 2017, 2017, 3517–3527. 10.1002/ejoc.201700121. - DOI - PMC - PubMed
-
For a review on de-amidative cross-coupling see:
- Liu C.; Szostak M. Chem. - Eur. J. 2017, 23, 7157–7173. 10.1002/chem.201605012. - DOI - PubMed
-
-
-
For the coupling of benzoic acids with arenes see:
- Voutchkova A.; Coplin A.; Leadbeater N. E.; Crabtree R. H. Chem. Commun. 2008, 6312–6314. 10.1039/b813998a. - DOI - PubMed
- Wang C.; Piel I.; Glorius F. J. Am. Chem. Soc. 2009, 131, 4194–4195. 10.1021/ja8100598. - DOI - PubMed
- Cornella J.; Lu P.; Larrosa I. Org. Lett. 2009, 11, 5506–5509. 10.1021/ol902304n. - DOI - PubMed
- Zhou J.; Hu P.; Zhang M.; Huang S.; Wang M.; Su W. Chem. - Eur. J. 2010, 16, 5876–5881. 10.1002/chem.201000529. - DOI - PubMed
- Xie K.; Yang Z.; Zhou X.; Li X.; Wang S.; Tan Z.; An X.; Guo C.-C. Org. Lett. 2010, 12, 1564–1567. 10.1021/ol100296b. - DOI - PubMed
- Zhang F.; Greaney M. F. Angew. Chem., Int. Ed. 2010, 49, 2768–2771. 10.1002/anie.200906921. - DOI - PubMed
- Zhao H.; Wei Y.; Xu J.; Kan J.; Su W.; Hong M. J. Org. Chem. 2011, 76, 882–893. 10.1021/jo102175f. - DOI - PubMed
- Hu P.; Zhang M.; Jie X.; Su W. Angew. Chem., Int. Ed. 2012, 51, 227–231. 10.1002/anie.201106451. - DOI - PubMed
- Luo H.-Q.; Dong W.; Loh T.-P. Tetrahedron Lett. 2013, 54, 2833–2836. 10.1016/j.tetlet.2013.03.086. - DOI
- Pei K.; Jie X.; Zhao H.; Su W. Eur. J. Org. Chem. 2014, 2014, 4230–4233. 10.1002/ejoc.201402278. - DOI
- Suresh R.; Muthusubramanian S.; Kumaran R. S.; Manickam G. Asian J. Org. Chem. 2014, 3, 604–608. 10.1002/ajoc.201400013. - DOI
- Yang K.; Wang P.; Zhang C.; Kadi A. A.; Fun H.-K.; Zhang Y.; Lu H. Eur. J. Org. Chem. 2014, 2014, 7586–7589. 10.1002/ejoc.201403234. - DOI
- Chen L.; Ju L.; Bustin K. A.; Hoover J. M. Chem. Commun. 2015, 51, 15059–15062. 10.1039/C5CC06645J. - DOI - PubMed
- Zhao S.; Liu Y.-J.; Yan S.-Y.; Chen F.-J.; Zhang Z.-Z.; Shi B.-F. Org. Lett. 2015, 17, 3338–3341. 10.1021/acs.orglett.5b01560. - DOI - PubMed
- Kan J.; Huang S.; Lin J.; Zhang M.; Su W. Angew. Chem., Int. Ed. 2015, 54, 2199–2203. 10.1002/anie.201408630. - DOI - PubMed
- Patra T.; Nandi S.; Sahoo S. K.; Maiti D. Chem. Commun. 2016, 52, 1432–1435. 10.1039/C5CC08367B. - DOI - PubMed
- Candish L.; Freitag M.; Gensch T.; Glorius F. Chem. Sci. 2017, 8, 3618–3622. 10.1039/C6SC05533H. - DOI - PMC - PubMed
-
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

