TFOS DEWS II pain and sensation report
- PMID: 28736339
- PMCID: PMC5706540
- DOI: 10.1016/j.jtos.2017.05.002
TFOS DEWS II pain and sensation report
Abstract
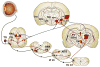
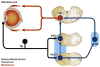
Pain associated with mechanical, chemical, and thermal heat stimulation of the ocular surface is mediated by trigeminal ganglion neurons, while cold thermoreceptors detect wetness and reflexly maintain basal tear production and blinking rate. These neurons project into two regions of the trigeminal brain stem nuclear complex: ViVc, activated by changes in the moisture of the ocular surface and VcC1, mediating sensory-discriminative aspects of ocular pain and reflex blinking. ViVc ocular neurons project to brain regions that control lacrimation and spontaneous blinking and to the sensory thalamus. Secretion of the main lacrimal gland is regulated dominantly by autonomic parasympathetic nerves, reflexly activated by eye surface sensory nerves. These also evoke goblet cell secretion through unidentified efferent fibers. Neural pathways involved in the regulation of meibomian gland secretion or mucin release have not been identified. In dry eye disease, reduced tear secretion leads to inflammation and peripheral nerve damage. Inflammation causes sensitization of polymodal and mechano-nociceptor nerve endings and an abnormal increase in cold thermoreceptor activity, altogether evoking dryness sensations and pain. Long-term inflammation and nerve injury alter gene expression of ion channels and receptors at terminals and cell bodies of trigeminal ganglion and brainstem neurons, changing their excitability, connectivity and impulse firing. Perpetuation of molecular, structural and functional disturbances in ocular sensory pathways ultimately leads to dysestesias and neuropathic pain referred to the eye surface. Pain can be assessed with a variety of questionaires while the status of corneal nerves is evaluated with esthesiometry and with in vivo confocal microscopy.
Keywords: Central nervous system; Cold receptors; Corneal esthesiometry; Dry eye disease; In vivo confocal microscopy; Mechano-nociceptors; Neuropathic pain; Ocular surface dryness; Pain; Peripheral sensory nerves; Polymodal nociceptors; Sensation; TRP channels; Trigeminal brainstem nuclear complex.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures




References
-
- Stapleton F, Marfurt C, Golebiowski B, Rosenblatt M, Bereiter D, Begley C, et al. TFOS international Workshop on contact lens discomfort, the TFOS international Workshop on contact lens discomfort: report of the subcommittee on neurobiology. Investig Ophthalmol Vis Sci. 2013;54(11):TFOS71–TFOS97. - PMC - PubMed
-
- Belmonte C, Tervo TT. Pain in and around the eye. In: McMahon SB, Koltzenburg M, Tracey I, Turks DC, editors. Wall and Melzack’s Textbook of Pain. 6. Philadelphia, PA, USA: Elsevier Saunders; 2013.
-
- Wilson N. The semantics of pain in Greco-Roman antiquity. J Hist Neurosci. 2013;22(2):129–143. - PubMed
-
- IASP. The International Association for the Study of Pain
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

