Estimating the cost-effectiveness of daclatasvir + sofosbuvir versus sofosbuvir + ribavirin for patients with genotype 3 hepatitis C virus
- PMID: 28736505
- PMCID: PMC5521139
- DOI: 10.1186/s12962-017-0077-4
Estimating the cost-effectiveness of daclatasvir + sofosbuvir versus sofosbuvir + ribavirin for patients with genotype 3 hepatitis C virus
Abstract
Background: As treatments for chronic hepatitis C are moving away from interferon-containing regimens, the most appropriate allocation of resources to higher cost, interferon-free, direct-acting antiviral (DAA) regimens needs to be assessed. Hepatitis C virus (HCV) genotype 3 is associated with faster disease progression and has fewer treatment options, historically, than other HCV genotypes. This analysis aims to estimate the comparative cost-effectiveness of two recently licenced interferon-free regimens for the treatment of HCV genotype 3.
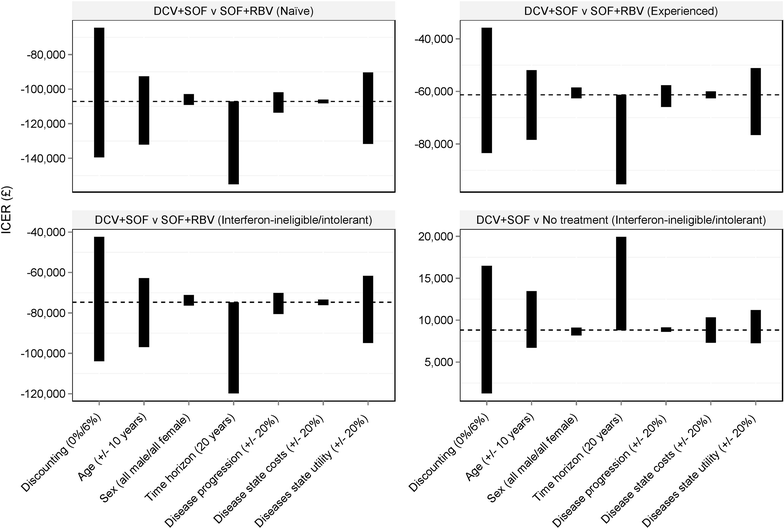
Methods: Utilising a published Markov model and results of a matching-adjusted indirect comparison of recently published clinical trial data (ALLY-3 and VALENCE, respectively), 12 weeks of treatment with daclatasvir + sofosbuvir (DCV + SOF) was compared to 24 weeks of treatment with sofosbuvir + ribavirin (SOF + RBV). UK-specific model inputs were used to inform a cost-utility analysis of these regimens.
Results: In the base case analysis, DCV + SOF was found to be dominant over SOF + RBV in treatment-naïve patients, patients that had previously been treated, and patients that are intolerant to, or ineligible for, interferon-containing regimens. Given the low rates of treatment currently observed in the UK, DCV + SOF was also compared to no treatment in the interferon-ineligible/intolerant patients, and may be considered cost-effective with an incremental cost-effectiveness ratio of £8817.
Conclusions: When compared to 24 weeks of SOF + RBV, 12 weeks of treatment with DCV + SOF results in improved quality of life and reduced total costs, and therefore is likely to represent significant clinical and economic value as a treatment option for genotype 3 HCV infection.
Keywords: Cost-effectiveness; Daclatasvir; Hepatitis C virus; Sofosbuvir.
Figures
References
-
- Public Health England, Health Protection Scotland, Public Health Wales, Public Health Agency. Hepatitis C in the UK: 2014 report. 2014. https://www.gov.uk/government/uploads/system/uploads/attachment_data/fil.... Accessed Jan 2015.
-
- European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C. 2014. http://files.easl.eu/easl-recommendations-on-treatment-of-hepatitis-C.pdf. Accessed Sep 2014. - PubMed
-
- World Health Organization. Guidelines for the screening, care and treatment of persons with hepatitis C infection. 2014 http://www.who.int/hiv/pub/hepatitis/hepatitis-c-guidelines/en/. Accessed Jan 2015. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials