Depletion of Pax7+ satellite cells does not affect diaphragm adaptations to running in young or aged mice
- PMID: 28736900
- PMCID: PMC5621498
- DOI: 10.1113/JP274611
Depletion of Pax7+ satellite cells does not affect diaphragm adaptations to running in young or aged mice
Abstract
Key points: Satellite cell depletion does not affect diaphragm adaptations to voluntary wheel running in young or aged mice. Satellite cell depletion early in life (4 months of age) has minimal effect on diaphragm phenotype by old age (24 months). Prolonged satellite cell depletion in the diaphragm does not result in excessive extracellular matrix accumulation, in contrast to what has been reported in hind limb muscles. Up-regulation of Pax3 mRNA+ cells after satellite cell depletion in young and aged mice suggests that Pax3+ cells may compensate for a loss of Pax7+ satellite cells in the diaphragm. Future investigations should focus on the role of Pax3+ cells in the diaphragm during adaptation to exercise and ageing.
Abstract: Satellite cell contribution to unstressed diaphragm is higher compared to hind limb muscles, which is probably attributable to constant activation of this muscle to drive ventilation. Whether satellite cell depletion negatively impacts diaphragm quantitative and qualitative characteristics under stressed conditions in young and aged mice is unknown. We therefore challenged the diaphragm with prolonged running activity in the presence and absence of Pax7+ satellite cells in young and aged mice using an inducible Pax7CreER -R26RDTA model. Mice were vehicle (Veh, satellite cell-replete) or tamoxifen (Tam, satellite cell-depleted) treated at 4 months of age and were then allowed to run voluntarily at 6 months (young) and 22 months (aged). Age-matched, cage-dwelling, Veh- and Tam-treated mice without wheel access served as activity controls. Diaphragm muscles were analysed from young (8 months) and aged (24 months) mice. Satellite cell depletion did not alter diaphragm mean fibre cross-sectional area, fibre type distribution or extracellular matrix content in young or aged mice, regardless of running activity. Resting in vivo diaphragm function was also unaffected by satellite cell depletion. Myonuclear density was maintained in young satellite cell-depleted mice regardless of running, although it was modestly reduced in aged sedentary (-7%) and running (-19%) mice without satellite cells (P < 0.05). Using fluorescence in situ hybridization, we detected higher Pax3 mRNA+ cell density in both young and aged satellite cell-depleted diaphragm muscle (P < 0.05), which may compensate for the loss of Pax7+ satellite cells.
Keywords: Pax3; Pax7; fluorescent in-situ hybridization.
© 2017 The Authors. The Journal of Physiology © 2017 The Physiological Society.
Figures
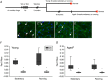
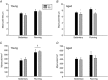



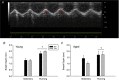

Comment in
-
The role of satellite cells in activity-induced adaptations: breathing new life into the debate.J Physiol. 2017 Oct 1;595(19):6225-6226. doi: 10.1113/JP274969. Epub 2017 Aug 29. J Physiol. 2017. PMID: 28802006 Free PMC article. No abstract available.
Similar articles
-
Satellite cell depletion does not affect diaphragm adaptations to hypoxia.J Appl Physiol (1985). 2022 Sep 1;133(3):637-646. doi: 10.1152/japplphysiol.00083.2022. Epub 2022 Jul 21. J Appl Physiol (1985). 2022. PMID: 35861521 Free PMC article.
-
Reduced voluntary running performance is associated with impaired coordination as a result of muscle satellite cell depletion in adult mice.Skelet Muscle. 2015 Nov 16;5:41. doi: 10.1186/s13395-015-0065-3. eCollection 2015. Skelet Muscle. 2015. PMID: 26579218 Free PMC article.
-
Depletion of resident muscle stem cells negatively impacts running volume, physical function, and muscle fiber hypertrophy in response to lifelong physical activity.Am J Physiol Cell Physiol. 2020 Jun 1;318(6):C1178-C1188. doi: 10.1152/ajpcell.00090.2020. Epub 2020 Apr 22. Am J Physiol Cell Physiol. 2020. PMID: 32320286 Free PMC article.
-
Skeletal muscle progenitor cells and the role of Pax genes.C R Biol. 2007 Jun-Jul;330(6-7):530-3. doi: 10.1016/j.crvi.2007.03.015. Epub 2007 Jun 13. C R Biol. 2007. PMID: 17631448 Review.
-
The molecular regulation of muscle stem cell function.Cold Spring Harb Symp Quant Biol. 2008;73:323-31. doi: 10.1101/sqb.2008.73.064. Epub 2009 Mar 27. Cold Spring Harb Symp Quant Biol. 2008. PMID: 19329572 Review.
Cited by
-
Hepatocyte growth factor acts as a mitogen for equine satellite cells via protein kinase C δ-directed signaling.J Anim Sci. 2018 Sep 7;96(9):3645-3656. doi: 10.1093/jas/sky234. J Anim Sci. 2018. PMID: 29917108 Free PMC article.
-
Fusion and beyond: Satellite cell contributions to loading-induced skeletal muscle adaptation.FASEB J. 2021 Oct;35(10):e21893. doi: 10.1096/fj.202101096R. FASEB J. 2021. PMID: 34480776 Free PMC article. Review.
-
Muscle from aged rats is resistant to mechanotherapy during atrophy and reloading.Geroscience. 2021 Feb;43(1):65-83. doi: 10.1007/s11357-020-00215-y. Epub 2020 Jun 25. Geroscience. 2021. PMID: 32588343 Free PMC article.
-
All for One and One for All: Regenerating Skeletal Muscle.Cold Spring Harb Perspect Biol. 2022 Aug 1;14(8):a040824. doi: 10.1101/cshperspect.a040824. Cold Spring Harb Perspect Biol. 2022. PMID: 34750174 Free PMC article. Review.
-
The β3-adrenergic receptor agonist mirabegron improves glucose homeostasis in obese humans.J Clin Invest. 2020 May 1;130(5):2319-2331. doi: 10.1172/JCI134892. J Clin Invest. 2020. PMID: 31961829 Free PMC article. Clinical Trial.
References
-
- Brack AS, Bildsoe H & Hughes SM (2005). Evidence that satellite cell decrement contributes to preferential decline in nuclear number from large fibres during murine age‐related muscle atrophy. J Cell Sci 118, 4813–4821. - PubMed
-
- Carlson BM (1973). The regeneration of skeletal muscle. A review. Am J Anat 137, 119–149. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

