Diverse roles for glycosaminoglycans in neural patterning
- PMID: 28736980
- PMCID: PMC5866094
- DOI: 10.1002/dvdy.24555
Diverse roles for glycosaminoglycans in neural patterning
Abstract
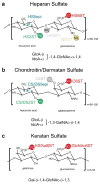
The nervous system coordinates the functions of most multicellular organisms and their response to the surrounding environment. Its development involves concerted cellular interactions, including migration, axon guidance, and synapse formation. These processes depend on the molecular constituents and structure of the extracellular matrices (ECM). An essential component of ECMs are proteoglycans, i.e., proteins containing unbranched glycan chains known as glycosaminoglycans (GAGs). A defining characteristic of GAGs is their enormous molecular diversity, created by extensive modifications of the glycans during their biosynthesis. GAGs are widely expressed, and their loss can lead to catastrophic neuronal defects. Despite their importance, we are just beginning to understand the function and mechanisms of GAGs in neuronal development. In this review, we discuss recent evidence suggesting GAGs have specific roles in neuronal patterning and synaptogenesis. We examine the function played by the complex modifications present on GAG glycans and their roles in regulating different aspects of neuronal patterning. Moreover, the review considers the function of proteoglycan core proteins in these processes, stressing their likely role as co-receptors of different signaling pathways in a redundant and context-dependent manner. We conclude by discussing challenges and future directions toward a better understanding of these fascinating molecules during neuronal development. Developmental Dynamics 247:54-74, 2018. © 2017 Wiley Periodicals, Inc.
Keywords: axon guidance; cell migration; chondroitin sulfate; code; dermatan sulfate; glycosaminoglycans; heparan sulfate; keratan sulfate; neuron; synapse.
© 2017 Wiley Periodicals, Inc.
Figures



References
-
- Antonini A, Stryker MP. Rapid remodeling of axonal arbors in the visual cortex. Science. 1993;260:1819–1821. - PubMed
-
- Aono S, Tokita Y, Shuo T, Yamauchi S, Matsui F, Nakanishi K, Hirano K, Sano M, Oohira A. Glycosylation site for chondroitin sulfate on the neural part-time proteoglycan, neuroglycan C. J Biol Chem. 2004;279:46536–46541. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

