Quantification of human brain PDE4 occupancy by GSK356278: A [11C](R)-rolipram PET study
- PMID: 28737056
- PMCID: PMC6238179
- DOI: 10.1177/0271678X17720868
Quantification of human brain PDE4 occupancy by GSK356278: A [11C](R)-rolipram PET study
Abstract
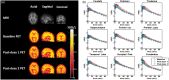
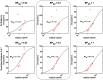
We characterized the relationship between the plasma concentration of the phospodiesterase (PDE)-4 inhibitor GSK356278 and occupancy of the PDE4 enzyme in the brain of healthy volunteers, using the positron emission tomography (PET) tracer [11C](R)-rolipram. To this end, PET scans were acquired in eight male volunteers before and at 3 and 8 h after a single 14 mg oral dose of GSK356278. A metabolite-corrected arterial input function was used in conjunction with the dynamic PET emission data to estimate volumes of distribution (VT) from a two-tissue compartment model. The administration of GSK356278 reduced [11C](R)-rolipram whole brain VT by 17% at 3 h post-dose (p = 0.01) and by 4% at 8 h post-dose. The mean plasma Cmax was 42.3 ng/ml, leading to a PDE4 occupancy of 48% at Tmax. The in vivo affinity of GSK356278 was estimated as EC50 = 46 ± 3.6 ng/ml. We present the first report of a direct estimation of PDE4 blockade in the living human brain. In vivo affinity of GSK356278 for the PDE4, estimated in this early phase study, was combined with GSK356278 safety and tolerability data to decide on a therapeutic dose for future clinical development.
Keywords: GSK356278; PDE4; PET; [11C](R)-rolipram; quantitative imaging.
Figures


References
-
- DaSilva JN, Lourenco CM, Meyer JH, et al. Imaging cAMP-specific phosphodiesterase-4 in human brain with R-[11C]rolipram and positron emission tomography. Eur J Nucl Med Mol Imaging 2002; 29: 1680–1683. - PubMed
-
- Matthews JC, Passchier J, Wishart MO, et al. The characterisation of both the R and S enantiomers of rolipram in man. J Cereb Blood Flow Metab 2003; 23(suppl): 678J.
-
- Parker CA, Matthews JC, Gunn RN, et al. Behaviour of [11C]R(-)- and [11C]S(+)-rolipram in vitro and in vivo, and their use as PET radiotracers for the quantificative assay of PDE4. Synapse 2005; 55: 270–279. - PubMed
-
- Guercio G, Castoldi D, Giubellina N, et al. Overall synthesis of GSK356278: quick delivery of a PDE4 inhibitor using a fit-for-purpose approach. Org Process Res Dev 2010; 14: 1153–1161.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

