Cell biology of spinal cord injury and repair
- PMID: 28737515
- PMCID: PMC5669582
- DOI: 10.1172/JCI90608
Cell biology of spinal cord injury and repair
Abstract
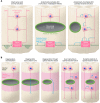
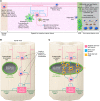
Spinal cord injury (SCI) lesions present diverse challenges for repair strategies. Anatomically complete injuries require restoration of neural connectivity across lesions. Anatomically incomplete injuries may benefit from augmentation of spontaneous circuit reorganization. Here, we review SCI cell biology, which varies considerably across three different lesion-related tissue compartments: (a) non-neural lesion core, (b) astrocyte scar border, and (c) surrounding spared but reactive neural tissue. After SCI, axon growth and circuit reorganization are determined by neuron-cell-autonomous mechanisms and by interactions among neurons, glia, and immune and other cells. These interactions are shaped by both the presence and the absence of growth-modulating molecules, which vary markedly in different lesion compartments. The emerging understanding of how SCI cell biology differs across lesion compartments is fundamental to developing rationally targeted repair strategies.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

